Table of contents
Introduction
Respondent Verification Component
Consent Component
Consent (CON)
Report (REP)
Urgent Condition (URG)
Screening Component
Screening Component Introduction (SCI)
Adherence to Guidelines (ATG)
Physical and Health Conditions (PHC)
Spirometry Questions (SPQ)
Medications and Health Remedies (MHR)
Physical Activity Readiness (PAR)
Other Reason for Screening Out (ORS)
Urine Collection Component
Introduction (UCI)
Urine Collection (URC)
Anthropometric Component
Anthropometric Component Introduction (ACI)
Height and Weight Measurement (HWM)
Skinfold Measurement (SFM)
Blood Pressure Component
Blood Pressure Measurement (BPM)
Phlebotomy Component
Phlebotomy Component Introduction (PHI)
Blood Collection (BDC)
Activity Monitor Component
Activity Monitor (AM)
Spirometry Component
Spirometry Restriction (SPR)
Spirometry Measurement (SPM)
mCAFT Component
mCAFT Measurement (AFT)
Grip Strength Component
Grip Strength Component Introduction (GSI)
Grip Strength Measurement (GSM)
Sit and Reach Component
Sit and Reach Component Introduction (SRI)
Sit and Reach Measurement (SRM)
Partial Curl-Up Component
Partial Curl-Up Component Introduction (PCI)
Partial Curl-Up Measurement (PCM)
Oral Health Component
Oral Health Component Introduction (OHI)
Oral Health Questions (OHQ)
Oral Health Restriction (OHR)
Oral Health Examination (OHE)
Lab Component
Report of Measurements
Exit Component
Exit Component Introduction (ECI)
Exit Consent Questions (ECQ)
Appendix I - Respondent verification form
Appendix II - Consent forms
English Assent Form for respondents 6 – 13
English Consent Form for parents of respondents 6 – 13
English Consent Form for respondents 14 – 19 (with storage)
English Consent Form for respondents 20+ (with storage)
Appendix III ― PAR-Q
Appendix IV- Spirometry Predicted Norms
Appendix V― Sample Report of Measurements
Appendix VI― Sample Letters to Health Care Provider
The following conventions are used in this document:
- Question text in bold font is read to the respondent. Text in normal font is not read to the respondent. Instructions to the person asking the questions or taking the measures are prefaced by the word “Instruction”, and are not read aloud.
- Question text in bold font enclosed by brackets () is read to the respondent at the discretion of the person asking the questions.
- In this text, the use of the masculine is generic and applies to both men and women. Please note that during the actual interview, the questions were personalized to be appropriate to the gender of the respondent.
- Text enclosed by square brackets [] is dynamic and may or may not appear on the computer screen based on the age, sex or other characteristics of the respondent.
- The options “Don’t Know” (DK) and “Refusal” (RF) are allowed on every question/measure unless otherwise stated. However, these response categories are shown in this document only when the flow from these responses is not to the next question/measure.
Upon arrival at the mobile clinic, the respondent is logged into the clinic by the clinic coordinator, which involves the following steps.
RVB_N11
Instruction: Press <1> to print the Respondent Verification Sheet.
1 Print the form
Note: For a sample respondent verification sheet, see Appendix I.
RVB_R11
Here is the first of several forms we will be asking you to complete. Please read it carefully and provide the information requested.
Instruction: Provide the respondent with the Respondent Verification Sheet.
When the respondent returns the form, check to ensure that all requested information has been filled in and is legible.
Note: Don’t Know and Refused are not allowed.
RVB_N12
Instruction: Press <1> to print the labels with the respondent’s CLINICID as a bar code identifier.
Attach the first label to a bracelet, and place the bracelet around the wrist of the respondent.
Attach the second label to the Physical Activity Readiness Questionnaire (PAR-Q) form.
1 Print the labels
Note: Don’t Know and Refused are not allowed.
NSC_N16
Instruction: Press <1> to print the labels with the respondent’s short name.
Attach the first label to the respondent's file folder.
Attach the second label to a urine sample container, and give the container to the respondent.
1 Print the label
Note: Don’t Know and Refused are not allowed.
Note: The respondent’s full name, gender, date of birth and their preferred official language are confirmed or updated using information from the Respondent Verification Form (appendix I).
RVC_END
To be completed by all respondents.
CON_N01
Instruction: Press <1> to print the Consent form(s).
1 Print the form(s)
Note: For respondents aged 6-14 print the assent and consent forms (see Appendix II).
Note: For respondents aged 14-19 print the consent (no DNA) form (see Appendix II).
Note: For respondents aged 20-79 print the consent form (see Appendix II).
CON_Q11
Before we start the clinic tests, we need to review the consent booklet that was given to you during the interview at your home. Did you have a chance to read that booklet?
Instruction: Show the respondent the consent package.
- Yes
- No (Go to CON_R13)
Note: Don’t Know and Refused are not allowed.
All respondents
CON_R12
Do you have any questions about any of the information in the consent booklet or about the clinic portion of the survey?
Instruction: Answer any questions as thoroughly as possible
Note: Don’t Know and Refused are not allowed.
Go to CON_R14
CON_R13
Here is a copy of the booklet. Please take a few minutes to read through it. If you have any questions about the information in the booklet or the clinic tests, I can answer them for you.
Instruction: Hand the consent package to the respondent and give them time to read through it (approximately 5 minutes).
Note: Don’t Know and Refused are not allowed.
CON_R14
Here is the Consent form for participation in the clinic portion of the survey. Please read the form carefully and mark either the “Yes” or “No” box for each item.
Instruction: Provide [the parent or guardian/the respondent] with the Consent form.
When [the parent or guardian/the respondent] returns the form, check to ensure that it has been completed correctly.
Sign and date the form as the witness.
Note: Don’t Know and Refused are not allowed.
If respondent is 14 or older, go to CON_N16.
CON_N15
Instruction: Record whether a parent or guardian has consented to the respondent participating in the physical measure tests.
- Yes
- No (Go to CON_END)
Note: Don’t Know and Refused are not allowed.
Respondents aged 13 and under
CON_R16
Your parent or guardian has said you can take part in the tests today. If you would like to participate we need you to write or print your name on this form.
Instruction: Provide the child with the Assent form.
When the child returns the form, check to ensure that it has been completed correctly.
Sign and date the form as the witness.
Note: Don’t Know and Refused are not allowed.
CON_N16
Instruction: Record whether the respondent has consented to participating in the physical measure tests.
- Yes
- No (Go to CON_END)
Note: Don’t Know and Refused are not allowed.
All respondents
CON_N21
Instruction: Record whether a Report of Laboratory Tests has been requested.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
If respondent’s age is 6 to 13, go to CON_N24.
CON_N23
Instruction: Record whether Statistics Canada has been authorised to provide information regarding Hepatitis B and C to the appropriate provincial authority.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 14 and over
If respondent has requested a Report of Laboratory Tests or if the respondent has declined to share Hepatitis B and C results with the appropriate provincial authority or if CON_AGE > 13, go to CON_N25.
CON_R23
You have indicated on your consent form that you do not want to receive a copy of your laboratory test results. However, you have agreed that Statistics Canada can test your blood for Hepatitis B and C. I just want you to be aware that, by agreeing to have the Hepatitis B and C tests done, you will receive the results if you test positive.
Instruction: Answer any questions as thoroughly as possible.
Note: Don’t Know and Refused are not allowed.
If respondent is 14 or older, go to CON_N25.
CON_N24
Instruction: Record whether a parent or guardian has consented to storage of the respondent’s blood and urine.
- Yes
- No (Go to CON_END)
Note: Don’t Know and Refused are not allowed.
Respondents aged 13 and under
CON_N25
Instruction: Record whether the respondent has agreed to storage of blood and urine.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
All respondents excluding those who did not consent to storage of blood and urine [not CON_Q24 = 2] If respondent is 19 or younger, go to CON_END.
CON_N26
Instruction: Record whether the respondent has agreed to storage of DNA.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 20 and over
CON_END
If the parent or guardian has not consented to the respondent participating in the physical measures tests or if the respondent has not consented to the physical measures tests, go to REP_END. If the respondent has declined a Report of Laboratory Tests, or if no mailing address exists (i.e., the street and city fields in the mailing address are empty), go to REP_B22.
REP_R11
You will receive a copy of [your/[name of respondent]’s] physical measurement test results at the end of the clinic visit today but we will not have the results of the blood and urine tests for about 8-12 weeks.
Note: Don’t Know and Refused are not allowed.
REP_Q12
What delivery method would you prefer?
Instruction: Read categories to respondent.
- Regular mail
- Courier
Note: Don’t Know and Refused are not allowed.
Respondents who want to receive a copy of their laboratory test results
If respondent is 14 or older or if no mailing address exists (i.e., the street and city fields in the mailing address are empty), go to REP_B22.
REP_N13
Instruction: Record the name of the person who signed the Consent form.
Enter the person’s first and last name.
Note: Don’t Know, Refused and Empty are not allowed.
If no mailing address exists (i.e., the street and city fields in the mailing address are empty), go to REP_B22.
REP_Q21
I would like to confirm your mailing address. Is it:
[Address]
- Yes (Go to REP_END)
- No
Note: Don’t Know and Refused are not allowed.
Respondents who have the street and city fields listed in the mailing address
REP_B22
What is your mailing address?
INSTRUCTION: Record the mailing address: civic number, street name, apartment number (if necessary), city, postal code and province.
Respondents who have the street and city fields in the mailing address blank
REP_END
If no telephone number exists, go to URG_B12.
URG_Q11
I would like to confirm your telephone number. Is it:
[Telephone Number]
- Yes (Go to URG_END)
- No
Note: Don’t Know and Refused are not allowed. Respondents who have a telephone number on file
URG_B12
What is your telephone number?
Instruction: Enter the area code and telephone number. Enter “000” if no telephone. Respondents who do not have a telephone number on file
URG_END
To be completed by all respondents.
SCI_R1
The following questions are asked to ensure that you are given all the tests for which you are eligible. Some questions may have been asked during the home interview, but we need to ensure that our information is up-to-date. We also need to know if any changes have occurred since the home interview. It is important to note that some medications and physical conditions may exclude you from certain tests.
Please answer to the best of your knowledge, as accurate information about you is important.
Note: If the respondent is younger than 14 then the following sentence is added:
Your parent or guardian may need to help you answer some of these questions .
Note: Don’t Know and Refused are not allowed.
SCI_END
ATG_R11
At the time of the home interview you were given a set of pre-testing guidelines. We will now review those guidelines.
Note: Don’t Know and Refused are not allowed.
ATG_Q11
When did you last eat or drink anything other than water?
Instruction: Enter the time followed by “AM” or “PM”. (insert respondent answer between 01:00 and 12:59)
Note: Don’t Know and Refused are not allowed.
All respondents
If difference between Appointment Time and ATG_Q11 is 10 hours or more or If CON_AGE > 69, go to ATG_Q21.
ATG_N12
Instruction: Probe to determine what and how much the respondent ate or drank. Record whether the respondent met the fasting requirements.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents who ate or drank something other than water less than 10 hours before their appointment time [ATG_Q11 -appointment time < 10 hours]
If respondent is older than 69, go to ATG_Q21.
ATG_N13
Instruction: Record whether the respondent should be screened out of the mCAFT.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 69 and under
ATG_Q21
Have you smoked cigarettes or used other tobacco or nicotine products during the past 2 hours?
- Yes
- No
All respondents
ATG_Q31
Have you consumed any alcohol since midnight?
- Yes
- No (Go to ATG_Q41)
Note: Don’t Know and Refused are not allowed.
All respondents
ATG_N32
Instruction: Probe to determine when and how much the respondent drank.
Record whether the respondent should be excluded from one or more tests.
- Yes
- No (Go to ATG_Q41)
Note: Don’t Know and Refused are not allowed.
Respondents who had consumed alcohol on the day of their appointment [ATG_Q31 = 1]
ATG_N33
From which tests should the respondent be excluded?
Instruction: Mark all that apply.
- Grip strength
- mCAFT
- Sit and reach
- Partial curl-ups
Note: Don’t Know and Refused are not allowed.
Respondent who had consumed alcohol on the day of their appointment and should be excluded from one or more tests [ATG_N32 = 1]
ATG_Q41
Have you exercised today? (e.g., running, swimming, weight training, etc.)
- Yes
- No (Go to ATG_END)
Note: Don’t Know and Refused are not allowed.
All respondents
ATG_Q42
For how long did you exercise?
- 1 to 15 minutes
- 16 to 30 minutes
- 31 to 60 minutes
- More than one hour
Respondents who had exercised on the day of their appointment [ATG_Q41 = 1]
ATG_END
PHC_R11
I am now going to ask you about your current health and physical condition.
Note: Don’t Know and Refused are not allowed.
If the respondent is male, or if the respondent is a female younger than 14 or older than 55, go to PHC_Q31.
PHC_Q11
Are you currently pregnant?
- Yes
- No
Note: Refused is not allowed.
Female respondents aged 14 to 55
If respondent is pregnant, go to PHC_Q12. Otherwise, go to PHC_Q31.
PHC_Q12
In what week are you?
(insert respondent answer between 1 and 45)
Female respondents aged 14 to 55 who are pregnant [PHC_Q11 = 1]
PHC_Q31
Have you been diagnosed with exercise induced asthma or a breathing condition that worsens with exercise? (For example: chronic bronchitis, emphysema, COPD.)
- Yes (Go to PHC_Q36)
- No
Note: Don’t Know and Refused are not allowed.
All respondents
If according to the household interview the respondent has been diagnosed with asthma, go to PHC_Q32. If CCC_Q41 = 1 (respondent has been diagnosed with chronic bronchitis), go to PHC_Q33. If CCC_Q43 = 1 (respondent has been diagnosed with emphysema), go to PHC_Q34. If CCC_Q45 = 1 (respondent has been diagnosed with chronic obstructive pulmonary disease), go to PHC_Q35. If PHC_Q31 = 1, go to PHC_Q36. Otherwise go to PHC_Q41.
PHC_Q32
During the interview in your home, it was reported that you had asthma. Is this correct?
- Yes (Go to PHC_Q36)
- No
Respondents who had previously reported being diagnosed with asthma [PHC_Q31 = 2 and CCC_Q11 = 1]
If according to the household interview the respondent has been diagnosed with chronic bronchitis, go to PHC_Q33. If CCC_Q43 = 1 (respondent has been diagnosed with emphysema), go to PHC_Q34. If CCC_Q45 = 1 (respondent has been diagnosed with chronic obstructive pulmonary disease), go to PHC_Q35. If PHC_Q31 = 1, go to PHC_Q36. Otherwise go to PHC_Q41.
PHC_Q33
During the interview in your home, it was reported that you had chronic bronchitis. Is this correct?
- Yes
- No
Respondents who had previously reported being diagnosed with bronchitis [PHC_Q31 = 2 and CCC_Q41 = 1]
If according to the household interview the respondent has been diagnosed with emphysema, go to PHC_Q34. If CCC_Q45 = 1 (respondent has been diagnosed with chronic obstructive pulmonary disease), go to PHC_Q35. If PHC_Q31 = 1, go to PHC_Q36. Otherwise go to PHC_Q41.
PHC_Q34
During the interview in your home, it was reported that you had emphysema. Is this correct?
- Yes
- No
Respondents who had previously reported being diagnosed with emphysema [PHC_Q31 = 2 and CCC_Q43 = 1]
If according to the household interview the respondent has been diagnosed with chronic obstructive pulmonary disease, go to PHC_Q35. If PHC_Q31 = 1, go to PHC_Q36. Otherwise go to PHC_Q41.
PHC_Q35
During the interview in your home, it was reported that you had chronic obstructive pulmonary disease (COPD). Is this correct?
- Yes
- No
Respondents who had previously reported being diagnosed with chronic obstructive pulmonary disease [PHC_Q31 = 2 and CCC_Q45 = 1]
If respondent has been diagnosed with exercise induced asthma or a breathing condition that worsens with exercise, go to PHC_Q36. Otherwise go to PHC_Q41.
PHC_Q36
Are you currently taking any medication for your breathing condition(s)?
- Yes
- No (Go to PHC_Q41)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported being diagnosed with any type of breathing condition [PHC_Q31 = 1 or PHC_Q32 = 1 or PHC_Q33 = 1 or PHC_Q34 = 1 or PHC_Q35 = 1]
If respondent is older than 69, go to PHC_Q41.
PHC_Q37
Do you have your medication with you?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 69 and under who had previously reported being diagnosed with any type of breathing condition [age < 70 and PHC_Q36 = 1]
PHC_Q41
Do you have an acute condition (e.g., sprained ankle, cold, flu, other infection) or chronic condition that may prevent you from participating in any of the tests today?
- Yes - Specify (insert respondent answer to a maximum of 80 characters)
- No (Go to PHC_Q51)
Note: Don’t Know and Refused are not allowed.
All respondents
PHC_N42
From which tests should the respondent be excluded because of this condition?
Instruction: Probe to determine the seriousness of the condition.
Mark all that apply.
- Phlebotomy
- Urine
- Height and Weight
- Skinfolds
- Activity monitor
- Spirometry
- mCAFT
- Grip strength
- Sit and reach
- Partial curl-ups
- Oral health
- None
Note: Don’t Know and Refused are not allowed.
Respondents with an acute or chronic condition that would prevent participation in clinic tests [PHC_41 = 1]
PHC_Q51
Do you have hemophilia?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
All respondents
PHC_Q52
Have you received chemotherapy in the past four weeks?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
All respondents
PHC_END
If respondent is older than 12, go to SPQ_R21.
SPQ_R11
The next set of questions is related to the health of [respondent’s first name]’s lungs.
Instruction: Ask the questions of the parent or guardian of the respondent.
Note: Don’t Know and Refused are not allowed.
SPQ_Q11
Has your child ever had wheezing or whistling in the chest at any time in the past?
- Yes
- No (Go to SPQ_Q16)
Note: Don’t Know and Refused are not allowed.
Parent or guardian of respondents aged 12 and under
SPQ_Q12
Has your child had wheezing or whistling in the chest in the last 12 months?
- Yes
- No (Go to SPQ_Q16)
Note: Don’t Know and Refused are not allowed.
Parent or guardian of respondent aged 12 and under who has ever had wheezing or whistling in the chest at any time in the past[SPQ_Q11 = 1]
SPQ_Q13
How many attacks of wheezing has your child had in the last 12 months?
Instruction: Read categories to respondent.
- 1 to 3 attacks
- 4 to 12 attacks
- More than 12 attacks
Note: Don’t Know and Refused are not allowed.
Parent or guardian of respondents aged 12 and under who has had wheezing or whistling in the chest in the last 12 months [SPQ_Q12 = 1]
SPQ_Q14
In the last 12 months, how often, on average, has your child’s sleep been disturbed due to wheezing?
Instruction: Read categories to respondent.
- Never woken with wheezing
- Less than one night per week
- One or more nights per week
Note: Don’t Know and Refused are not allowed.
Parent or guardian of respondents aged 12 and under who has had wheezing or whistling in the chest in the last 12 months [SPQ_Q12 = 1]
SPQ_Q15
In the last 12 months, has wheezing ever been severe enough to limit your child’s speech to only one or two words at a time between breaths?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Parent or guardian of respondents aged 12 and under who has had wheezing or whistling in the chest in the last 12 months [SPQ_Q12 = 1]
SPQ_Q16
In the last 12 months, has your child’s chest sounded wheezy during or after exercise?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Parent or guardian of respondents aged 12 and under
SPQ_Q17
In the last 12 months, has your child had a dry cough at night, apart from a cough associated with a cold or a chest infection?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Parent or guardian of respondents aged 12 and under
Go to SPQ_END.
SPQ_R21
The next set of questions is related to the health of your lungs.
Note: Don’t Know and Refused are not allowed.
SPQ_Q21
Do you cough regularly?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 13 and over
SPQ_Q22
Do you cough up phlegm regularly?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 13 and over
SPQ_Q23
Do even simple chores make you short of breath?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 13 and over
SPQ_Q24
Do you wheeze when you exert yourself, or at night?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 13 and over
SPQ_Q25
Do you get frequent colds that persist longer than those of other people you know?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 13 and over
SPQ_END
Prescription medications
If no prescription medications were reported in the household interview, go to MHR_Q121.
MHR_R100
Now I’d like to confirm your use of prescription medications.
Instruction: For each medication listed from the home interview, ask the following two questions.
Note: Don’t Know and Refused are not allowed.
CDP_Q1
During the interview in your home, it was reported that you were taking [name of prescription medication]. Are you still taking that medication?
- Yes
- No (Go to next medication or MHR_Q121)
- Never took the medication (Go to next medication or MHR_Q121)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking prescription medication
CDP_Q2
When was the last time that you took that medication?
Instruction: Read categories to respondent.
- Today
- Yesterday
- Within the last week
- Within the last month
- More than one month ago
Respondents who had previously reported that they were taking prescription medication
MHR_Q121
Are you taking any other prescription medications? (Remember to include prescribed medications such as insulin, nicotine patches and birth control (pills, patches or injections).)
- Yes
- No (Go to MHR_R200)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking prescription medication
MHR_Q122
How many?
(insert respondent answer between 1 and 95)
Instruction: For each other prescription medication, to a maximum of five, ask the following five questions.
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking prescription medication [MHR_Q121 = 1]
NDP_Q1
Is a Drug Identification Number (DIN) available for the medication?
Instruction: If necessary, help the respondent to find the DIN on the bottle, tube or box.
- Yes
- No (Go to NDP_Q4)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking prescription medication
NDP_Q2
What is the DIN of the medication?
Instruction: Record DIN from the bottle, tube or box. Be sure to use eight digits; use leading zeros to fill the field if necessary (e.g., 00012345).
(insert Drug Identification Number between 00000001 and 99999995)
Note: Don’t Know and Refused and Empty are not allowed.
Respondents who had previously reported that they were taking prescription medication
NDP_N3
Instruction: The name associated with DIN [number] is [medication name]. Please confirm.
- Yes (Go to NDP_Q5)
- No
Note: Don’t Know and Refused are not allowed.
NDP_Q4
What is the exact name and dosage of the medication?
Instruction: Record the exact name and dosage of the medication from the bottle, tube or box.
Note: Empty is not allowed.
Respondents who had previously reported that they were taking prescription medication
NDP_Q5
When was the last time that you took that medication?
Instruction: Read categories to respondent.
- Today
- Yesterday
- Within the last week
- Within the last month
- More than one month ago
Respondents who had previously reported that they were taking prescription medication
Over-the-Counter medications
If no over-the-counter medications were reported in the household interview, go to MHR_Q221.
MHR_R200
Now I’d like to confirm your use of over-the-counter medications.
Instruction: For each medication listed from the home interview, ask the following two questions.
Note: Don’t Know and Refused are not allowed.
CDP_Q1
During the interview in your home, it was reported that you were taking [name of over-the-counter medication]. Are you still taking that medication?
- Yes
- No (Go to next medication or MHR_Q221)
- Never took the medication (Go to next medication or MHR_Q221)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking over-the-counter medication
CDP_Q2
When was the last time that you took that medication?
Instruction: Read categories to respondent.
- Today
- Yesterday
- Within the last week
- Within the last month
- More than one month ago
Respondents who had previously reported that they were taking over-the-counter medication
MHR_Q221
Are you taking any other over-the-counter medications? (Pain killers, antacids, allergy pills and hydrocortisone creams are all examples of over-the-counter medications.)
- Yes
- No (Go to MHR_R300)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking over-the-counter medication
MHR_Q222
How many?
(insert respondent answer between 1 and 95)
Instruction: For each other over-the-counter medication, to a maximum of five, ask the following five questions.
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking over-the-counter medication [MHR_Q221 = 1]
NDP_Q1
Is a Drug Identification Number (DIN) available for the medication?
Instruction: If necessary, help the respondent to find the DIN on the bottle, tube or box.
- Yes
- No (Go to NDP_Q4)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking over-the-counter medication
NDP_Q2
What is the DIN of the medication?
Instruction: Record DIN from the bottle, tube or box. Be sure to use eight digits; use leading zeros to fill the field if necessary (e.g., 00012345).
(insert Drug Identification Number between 00000001 and 99999995)
Note: Don’t Know, Refused and Empty are not allowed.
Respondents who had previously reported that they were taking over-the-counter medication
NDP_N3
Instruction: The name associated with DIN [number] is [medication name]. Please confirm.
- Yes (Go to NDP_Q5)
- No
Note: Don’t Know and Refused are not allowed.
NDP_Q4
What is the exact name and dosage of the medication?
Instruction: Record the exact name and dosage of the medication from the bottle, tube or box.
Note: Empty is not allowed.
Respondents who had previously reported that they were taking over-the-counter medication
NDP_Q5
When was the last time that you took that medication?
Instruction: Read categories to respondent.
- Today
- Yesterday
- Within the last week
- Within the last month
- More than one month ago
Respondents who had previously reported that they were taking over-the-counter medication
Health product and herbal remedies
If no health product or herbal remedies were reported in household interview, go to MHR_Q321.
MHR_R300
Now I’d like to confirm your use of health products and herbal remedies.
Instruction: For each product or remedy listed from the home interview, ask the following two questions.
Note: Don’t Know and Refused are not allowed.
CDP_Q1
During the interview in your home, it was reported that you were taking [name of product or remedy]. Are you still taking that product?
- Yes
- No (Go to next product or MHR_Q321)
- Never took the product (Go to next product or MHR_Q321)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking a health product or herbal remedy
CDP_Q2
When was the last time that you took that product?
Instruction: Read categories to respondent.
- Today
- Yesterday
- Within the last week
- Within the last month
- More than one month ago
Respondents currently taking a health product or herbal remedy
MHR_Q321
Are you taking any other health products or herbal remedies such as vitamins, minerals, fish oils and other oils, and botanical or homeopathic preparations?
- Yes
- No (If CON_AGE < 14, go to MHR_N611. Otherwise go to MHR_R411)
Note: Don’t Know and Refused are not allowed.
Respondents who had previously reported that they were taking a health product or herbal remedy
MHR_Q322
How many?
(insert respondent answer between 1 and 95)
Instruction: For each other product or remedy, to a maximum of five, ask the following five questions.
Note: Don’t Know and Refused are not allowed.
Respondents currently taking a health product or herbal remedy [MHR_Q321 = 1]
NDP_Q1
Is a Drug Identification Number (DIN) available for the product?
Instruction: If necessary, help the respondent to find the DIN on the bottle, tube or box.
- Yes
- No (Go to NDP_Q4)
Note: Don’t Know and Refused are not allowed.
Respondents currently taking a health product or herbal remedy
NDP_Q2
What is the DIN of the product?
Instruction: Record DIN from the bottle, tube or box. Be sure to use eight digits; use leading zeros to fill the field if necessary (e.g., 00012345).
(insert Drug Identification Number between 00000001 and 99999995)
Note: Don’t Know, Refused and Empty are not allowed.
Respondents currently taking a health product or herbal remedy
NDP_N3
Instruction: The name associated with DIN [number] is [product name]. Please confirm.
- Yes (Go to NDP_Q5)
- No
Note: Don’t Know and Refused are not allowed.
NDP_Q4
What is the exact name and dosage of the product?
Instruction: Record the exact name and dosage of the product from the bottle, tube or box.
Note: Empty is not allowed.
Respondents currently taking a health product or herbal remedy
NDP_Q5
When was the last time that you took that product?
Instruction: Read categories to respondent.
- Today
- Yesterday
- Within the last week
- Within the last month
- More than one month ago
Respondents currently taking a health product or herbal remedy
If respondent is younger than 14, go to MHR_N611.
MHR_R411
Now I am going to ask you some questions about your use of other substances such as performance enhancing or recreational drugs. We ask these questions because these drugs can affect the results of the physical and biological measures that we will be taking today. You can be assured that anything you say will remain confidential.
Note: Don’t Know and Refused are not allowed.
MHR_Q411
In the past week have you used any performance enhancing or recreational drugs such as steroids, marijuana or cocaine?
- Yes
- No
Respondents aged 14 and over
MHR_N611
From which tests should the respondent be excluded because of medication use?
Instruction: Mark all that apply.
- Spirometry
- mCAFT
- Grip strength
- Sit and reach
- Partial curl-ups
- None
Note: Don’t Know and Refused are not allowed.
MHR_END
PAR_R01
For respondents 14 or older:
Next you need to complete a questionnaire called the Physical Activity Readiness Questionnaire. These questions are used to identify people for whom certain tests might be inappropriate. Please read the questionnaire and answer each question thinking about the tests that you will be doing today. If you have any questions please ask me. When you have completed the questionnaire, sign and date the bottom of the form.
Instruction: Provide the respondent with a blank PAR-Q (shown in Appendix III).
Show the laminated card with pictures of each testing component to the respondent.
Ensure that all PAR-Q questions have been answered.
Ensure that the respondent has signed and dated the form.
Sign and date the form as the witness.
For respondents younger than 14:
Next you need to complete a questionnaire called the Physical Activity Readiness Questionnaire. These questions are used to identify people for whom certain tests might be inappropriate. Your parent or guardian may need to help you read and answer some of these questions. If you have any questions please ask me. When you're done, please write or print your name at the bottom of this form.
Instruction: Provide the respondent with a blank PAR-Q (shown in Appendix III).
Show the laminated card with pictures of each testing component to the respondent.
Ensure that all PAR-Q questions have been answered.
Ask the parent or guardian to sign and date the form.
Sign and date the form as the witness.
Note: Don’t Know and Refused are not allowed.
PAR_R02
I am now going to enter that information into our computer system. I may have some additional questions about your responses.
Note: Don’t Know and Refused are not allowed.
PAR_N11
Has your doctor ever said that you have a heart condition and that you should only do physical activity recommended by a doctor?
Instruction: Enter the response from the PAR-Q completed by the respondent.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
All respondents
PAR_N21
Do you feel pain in your chest when you do physical activity?
Instruction: Enter the response from the PAR-Q completed by the respondent.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
All respondents
PAR_N31
In the past month, have you had chest pain when you were not doing physical activity?
Instruction: Enter the response from the PAR-Q completed by the respondent.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
All respondents
PAR_N41
Do you lose your balance because of dizziness or do you ever lose consciousness?
Instruction: Enter the response from the PAR-Q completed by the respondent.
- Yes
- No (Go to PAR_N51)
Note: Don’t Know and Refused are not allowed.
All respondents
PAR_Q42
In completing the questionnaire you reported that you lost your balance because of dizziness or have lost consciousness. Which condition was the reason for that response?
- Lost balance
- Lost consciousness
- Both
Respondents who previously reported losing their balance because of dizziness or losing consciousness [PAR_N41 = 1]
PAR_Q43
Was the last time that you [lost your balance/lost consciousness] within the last year?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
Respondents who previously reported losing their balance because of dizziness or losing consciousness [PAR_N41 = 1]
PAR_Q44
Under which condition(s) does this happen?
Instruction: Mark all that apply.
- Standing up quickly
- Getting up from lying down
- After an injury/accident (e.g., concussion, head injury)
- During an illness (e.g., inner ear infection)
- During or after exercise
- After fasting for a long period of time
- On hot days
- At random
- Other – Specify (insert respondent answer to a maximum of 80 characters)
Respondents who previously reported losing their balance because of dizziness or losing consciousness [PAR_N41 = 1]
If respondent has not lost balance or lost consciousness in the last year, go to PAR_N51.
If respondent has lost balance or lost consciousness in the last year and PAR_Q44 < 9, go to PAR_N51.
PAR_N45
Should the respondent be excluded from the mCAFT because of this condition?
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[PAR_Q43 = 1 and PAR_Q44 = 9]
PAR_N51
Do you have a bone or joint problem (for example back, knee or hip) that could be made worse by a change in your physical activity?
Instruction: Enter the response from the PAR-Q completed by the respondent.
- Yes
- No (Go to PAR_N61)
Note: Don’t Know and Refused are not allowed.
All respondents
PAR_Q52
In completing the questionnaire you reported that you have a bone or joint problem. The problem is with which bone or joint?
Instruction: Mark all that apply.
- Head / Jaw
- Neck
- Back / Spine (excluding neck)
- Shoulder
- Arm / Elbow
- Wrist
- Hand / Finger
- Hip
- Leg / Knee
- Ankle
- Foot / Toe
Respondents who previously reported having a bone or joint problem [PAR_N51 = 1]
PAR_B53A
For each bone or joint identified in question PAR_Q52, ask the following three questions:
BJP_Q1
What is the condition that affects your [bone or joint]
- Arthritis (osteoarthritis or rheumatoid arthritis)
- Vertebral disorder (e.g., chronic back or neck pain)
- Osteoporosis
- Chronic soft tissue condition (e.g., tendonitis)
- Chronic joint condition (e.g., bursitis, carpal tunnel syndrome)
- Acute soft tissue condition (e.g., pulled muscle, sprain, strain)
- Acute bone condition (e.g., broken bone)
- Neuromuscular disorder (e.g., multiple sclerosis, cerebral palsy, spinal cord dysfunction, muscular dystrophy, brain injury)
- Amputation
- Other – Specify (insert respondent answer to a maximum of 80 characters)
Respondents who previously reported having a bone or joint problem
For each bone or joint identified in question PAR_Q52, ask the following three questions:
BJP_Q2
What types of activities aggravate your [identified problem]?
Instruction: Probe as necessary to determine whether the respondent should be excluded from any physical tests.
Mark all that apply.
- Bending
- Lifting
- Climbing stairs
- Walking or running
- Squeezing
- Twisting
- Stretching or reaching
- Other – Specify (insert respondent answer to a maximum of 80 characters)
Respondents who previously reported having a bone or joint problem
BJP_N3
From which tests should the respondent be excluded because of this condition?
Instruction: Mark all that apply.
- mCAFT
- Grip strength
- Sit and reach
- Partial curl-ups
- None
Note: Don’t Know and Refused are not allowed
Respondents who previously reported having a bone or joint problem
PAR_N61
Is your doctor currently prescribing drugs (for example, water pills) for your blood pressure or a heart condition?
Instruction: Enter the response from the PAR-Q completed by the respondent.
- Yes
- No (Go to PAR_N71)
Note: A list of confirmed and new prescription drugs is displayed under a heading, “Medications Currently Being Taken.”
Note: Don’t Know and Refused are not allowed.
All respondents
PAR_Q62
For which condition(s) are you taking the drugs?
Instruction: Mark all that apply.
- High blood pressure
- Low blood pressure
- Angina
- Previous heart attack
- Aneurysm
- Arrhythmia
- Other heart condition – Specify (insert respondent answer to a maximum of 80 characters)
- Other medical condition – Specify (insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
Respondents currently taking prescription drugs [PAR_Q61 = 1]
PAR_N71
Do you know of any other reason why you should not do physical activity?
Instruction: Enter the response from the PAR-Q completed by the respondent.
- Yes – Specify (insert respondent answer to a maximum of 80 characters)
- No (Go to PAR_END)
Note: Don’t Know and Refused are not allowed.
All respondents
PAR_N72
From which tests should the respondent be excluded because of this condition?
Instruction: Probe to determine the seriousness of the condition.
Mark all that apply.
- mCAFT
- Grip strength
- Sit and reach
- Partial curl-ups
- None
Note: Don’t Know and Refused are not allowed.
[PAR_Q71 = 1]
PAR_END
ORS_Q1
Are there any other reasons why you should not participate in one or more of the physical tests?
- Yes
- No
All respondents
ORS_N1
Instruction: Is there any other reason why the respondent should not perform the Grip Strength test?
- Yes – Specify (insert respondent answer to a maximum of 80 characters)
- No
Note: Don’t Know and Refused are not allowed.
All respondents
ORS_N2
Instruction: Is there any other reason why the respondent should not perform the Spirometry test?
- Yes – Specify (insert respondent answer to a maximum of 80 characters)
- No
Note: Don’t Know and Refused are not allowed.
All respondents
If respondent is older than 69, go to ORS_END.
ORS_N3
Instruction: Is there any other reason why the respondent should not perform the modified Canadian Aerobic Fitness Test (mCAFT)?
- Yes – Specify (insert respondent answer to a maximum of 80 characters)
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 69 and under
ORS_N4
Instruction: Is there any other reason why the respondent should not perform the Sit and Reach test?
- Yes – Specify (insert respondent answer to a maximum of 80 characters)
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 69 and under
ORS_N5
Instruction: Is there any other reason why the respondent should not perform the Partial Curl-up test?
- Yes – Specify (insert respondent answer to a maximum of 80 characters)
- No
Note: Don’t Know and Refused are not allowed.
Respondents aged 69 and under
ORS_END
UCI_R01
Now we would like you to provide a urine sample. Please fill the cup up to the line and put the lid back on tightly. Once you are finished, place the filled cup in the brown paper bag and bring it back to this room. If you are unable to provide a sample at this time then we will try again later during the clinic visit.
Note: Don’t Know and Refused are not allowed.
UCI_END
URC_N01
Instruction: Record whether the respondent provided a urine sample.
- Yes (Go to URC_END)
- No
Note: Don’t Know and Refused are not allowed.
Note: Save the current time (for use in the Lab Component).
URC_N02
Instruction: Record the reason why the respondent did not provide a urine sample.
- Refusal
- Unable to provide
- Other – Specify (insert respondent answer to a maximum of 80 characters)
Note: Don’t Know and Refused are not allowed.
URC_END
ACI_R01
Next will be a series of body measurements.
Note: See Canadian Health Measures Survey Protocols for further details on measurement protocols and procedures.
Note: Don’t Know and Refused are not allowed.
All respondents
ACI_END
To be completed by all respondents except those meeting the exclusion criteria:
- The respondent has an acute condition that prevents him/her from completing the measure.
- Respondents who are unable to stand or sit unassisted.
HWM_Q11
I’m going to start by measuring how tall you are. Please remove your shoes and stand with your feet together and your heels, buttocks, back, and head in contact with the measuring device. Look straight ahead and stand as tall as possible. Now, take a deep breath in and hold it.
Instruction: Ensure the respondent’s head is in the Frankfort plane. Take the measurement while the breath is being held.
Note: Don’t Know and Refused are not allowed.
HWM_N11
Instruction: Record how the data will be captured.
- Electronically
- Manually (Go to HWM_N11B)
- Self-report (Go to HWM_N11B)
Note: Self-report data should only be recorded under specific circumstance (e.g. wheelchair bound, bun or hair piece that the respondent is unwilling to remove, etc.)
Note: Don’t Know and Refused are not allowed.
[PHC_Q42C = 2]
If captured Electronically:
HWM_N11A
Instruction: Ensure that the stadiometer is set to centimetres (cm). Press the “Send” button on the left side of the digital display box or the “Data” button on the SPC (send to PC) device. (insert stadiometer information between 700.00 and 2130.00 in millimetres)
Note: Don’t Know and Refused are not allowed.
If captured Manually or self-reported:
HWM_N11B
Instruction: Record the standing height in centimetres. (insert standing height between 70.00 and 213.00 in centimetres)
Don’t Know, Refused (Go to HWM_S11)
HWM_N11C
Instruction: Re-enter the standing height in centimetres. (insert standing height between 70.00 and 213.00 in centimetres)
HWM_S11
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
Self-reported: Go to HWM_R13
HWM_R12
Next I’d like you to sit on this box so that I can measure how tall you are when you are sitting. Sit with your back and head against the measuring device. Put your hands on your lap and keep your legs still. Look straight ahead and sit up as straight as possible. Now, take a deep breath in and hold it.
Instruction: Ensure the respondent’s head is in the Frankfort plane.
Ensure the respondent does not contract the gluteal muscles nor push with the legs.
Take the measurement while the breath is being held.
Note: Don’t Know and Refused are not allowed.
If captured Electronically:
HWM_N12A
Instruction: Ensure that the stadiometer is set to centimetres (cm).
Press the “Send” button on the left side of the digital display box or the “Data” button on the SPC (send to PC) device. (insert stadiometer information between 700.00 and 2130.00 in millimetres)
Note: Don’t Know and Refused are not allowed.
If captured Manually
HWM_N12B
Instruction: Record the sitting height in centimetres. (insert stadiometer information between 70.00 and 213.00 in centimetres)
Don’t Know, Refused (Go to HWM_S12)
HWM_N12C
Instruction: Re-enter the sitting height in centimetres. (insert stadiometer information between 70.00 and 213.00 in centimetres)
HWM_S12
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
HWM_R13
Next I’m going to measure how much you weigh. Please step onto the centre of the scale and face me. Keep your hands at your sides and look straight ahead.
Instruction: Ensure the respondent has on only minimal clothing (no shoes) and has nothing in his/her pockets.
Record in F4 – Remarks any exceptions to a normal weight measurement such as amputations, pregnancy, wheelchair, castings etc.
Note: Don’t Know and Refused are not allowed.
HWM_N13
Instruction: Record how the data will be captured.
- Electronically
- Manually (Go to HWM_N13B)
Note: Don’t Know and Refused are not allowed.
If captured Electronically:
HWM_N13A
Instruction: Ensure the scale is set to kilograms (kg). When the measurement is stable, press <Print> on the scale.
Press <1> to save the measurement in Blaise.
1 Save the measurement
If respondent is more than 12 weeks pregnant, to to SFM_END
If captured Manually:
HWM_N13B
Instruction: When the measurement is stable, record the weight. (insert measurement between 0.00 and 300.00 kilograms)
Don’t Know, Refused (Go to HWM_S13)
HWM_N13C
Instruction: Re-enter the weight in kilograms.
(insert measurement between 0.00 and 300.00)
If respondent is more than 12 weeks pregnant, to to SFM_END
HWM_S13
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
If respondent is more than 12 weeks pregnant, go to SFM_END.
HWM_R14
Now I’m going to measure your waist circumference. First I need to feel for your hip bones and for the bottom of your ribs. I will take the measurement between these two points. Please stand up straight with your arms hanging loosely at your sides, and breathe normally. I may need to move your clothing slightly because the measurement has to be taken directly on the skin. To ensure I have the correct position, I am going to make two small marks on your skin with a washable marker where the tape measure is to go. These marks will wash off with soap and water.
Instruction: Read the measurement at the side of the body. Take the measurement at the end of a normal expiration. If the respondent will not allow measurement on the skin, take the measurement over the shirt and use F4 – Remarks to make a note.
Note: Don’t Know and Refused are not allowed.
HWM_N14A
Instruction: Record the waist circumference. (insert measurement between 20.0 and 199.0 centimetres)
Don’t Know, Refused (Go to HWM_S14)
HWM_N14B
Instruction: Re-enter the waist circumference in centimetres. (insert measurement between 20.0 and 199.0)
HWM_S14
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
HWM_R15
Now I’m going to measure your hip circumference. Please stand up straight with your arms hanging loosely at your sides, and breathe normally. I may need to move your clothing slightly to ensure the measurement is accurate.
Instruction: Read the measurement at the side of the body. Take the measurement at the end of a normal expiration.
Note: Don’t Know and Refused are not allowed.
HWM_N15A
Instruction: Record the hip circumference. (insert measurement between 20.0 and 199.0 centimetres)
Don’t Know, Refused (Go to HWM_S15)
HWM_N15B
Instruction: Re-enter the hip circumference in centimetres. (insert measurement between 20.0 and 199.0)
HWM_S15
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
Then the following variables are calculated:
- body mass index
- body mass index norms for respondents 18 or older
- body mass index norms for respondents younger than 18
- waist circumference norms for respondents aged 15 to 69
- waist-to-hip ratio.
HWM_END
To be completed by all respondents except those meeting the exclusion criteria:
- Women who are more than 12 weeks pregnant.
- Respondents with BMI ≥ 30 kg/m2.
- Respondents with an acute condition that prevents them from completing the measure (e.g., varicose veins, skin condition).
SFM_Q01
In order to accurately calculate your body composition score I will also measure the thickness of your skinfolds using this skinfold caliper. To measure a skinfold I will take hold of a fold of skin plus any underlying fat tissue between my fingers. Then I will place the calipers on the fold at which time you may feel a slight pinch. Let me show you how it will feel on your hand.
Instruction: Show the skinfold calipers to the respondent and demonstrate the technique on the palm of the respondent’s hand. If respondent refuses, go to SFM_END.
Note: Don’t Know is not allowed.
All respondents except those meeting the exclusion criteria at the beginning of the Skinfold Measurement (SFM) block [PHC_Q42 = 4, excluding respondents with HWMDBMI > 29.99 and females who answered PHC_Q12>12]
SFM_R02
I will be measuring skinfolds at five sites: back of the arm (triceps), front of the arm (biceps), shoulder blade (subscapular), waist (iliac crest) and on the inside of your lower leg (medial calf). At each site I will be taking 2 or 3 measurements.
The measurement must be taken directly against the skin so I may need you to move your clothing slightly to have access to the various sites. First I need to mark the location of each site using this washable marker. The marks will wash off with soap and water.
Instruction: Mark all locations. Refer to the operations manual for the complete set of procedures.
Note: Don’t Know and Refused are not allowed.
First skinfold measurements
SFM_N11
Instruction: Record the first triceps skinfold measurement to the nearest 0.2 millimetres.
(insert measurement between 0.0 and 80.0)
Don’t Know, Refused (Go to SFM_S11)
All respondents, except those meeting the exclusion criteria at the beginning of the Anthropometric Component who answered PHC_Q42 = 4, excluding respondents with HWMDBMI > 29.99 and females who answered PHC_Q12 > 12
SFM_S11
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
SFM_N12
Instruction: Record the first biceps skinfold measurement to the nearest 0.2 millimetres.
(insert measurement between 0.0 and 80.0)
Don’t Know, Refused (Go to SFM_S12)
All respondents, except those meeting the exclusion criteria at the beginning of the Anthropometric Component who answered PHC_Q42 = 4, excluding respondents with HWMDBMI > 29.99 and females who answered PHC_Q12 > 12
SFM_S12
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
SFM_N13
Instruction: Record the first subscapular skinfold measurement to the nearest 0.2 millimetres.
(insert measurement between 0.0 and 80.0)
Don’t Know, Refused (Go to SFM_S13)
All Respondents, except those meeting the exclusion criteria at the beginning of the Anthropometric Component who answered PHC_Q42 = 4 , excluding respondents with HWMDBMI > 29.99 and females who answered PHC_Q12 > 12
SFM_S13
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
SFM_N14
Instruction: Record the first iliac crest skinfold measurement to the nearest 0.2 millimetres.
(insert measurement between 0.0 and 80.0)
Don’t Know, Refused (Go to SFM_S14)
All respondents, except those meeting the exclusion criteria at the beginning of the Anthropometric Component who answered PHC_Q42 = 4, excluding respondents with HWMDBMI > 29.99 and females who answered PHC_Q12 > 12
SFM_S14
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
SFM_N15
Instruction: Record the first medial calf skinfold measurement to the nearest 0.2 millimetres.
(insert measurement between 0.0 and 80.0)
Don’t Know, Refused (Go to SFM_S15)
All respondents, except those meeting the exclusion criteria at the beginning of the Anthropometric Component who answered PHC_Q42 = 4, excluding respondents with HWMDBMI > 29.99 and females who answered PHC_Q12 > 12
SFM_S15
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
Second skinfold measurements
Note: This sequence of measurements is repeated a second time and recorded for all five skinfold sites. If the difference between the two skinfold measures is greater than 0.4 mm at any site, or if one of the measurements could not be taken, a third measurement is taken for the site.
Third skinfold measurements
SFM_R30
The difference between the first and second measures at [the first/second/third/fourth/fifth site(s)] is too large, so I will have to take a third measurement for [this/these site(s)].
Note: The skinfold measurement(s) is/are retaken a third time for every site where the difference between the 1st and 2nd measurement is greater than 0.4 mm
Then the following variables are calculated:
- triceps skinfold average
- biceps skinfold average
- subscapular skinfold average
- iliac crest skinfold average
- medial calf skinfold average
- sum of five skinfolds.
- sum of five skinfolds norms for respondents 15 – 69
- body composition norms for respondents 15 – 69.
SFM_END
To be completed by all respondents except those meeting the exclusion criteria:
- Presence of the following on both arms: rashes, gauze dressings, casts, edema, paralysis, tubes, open sores or wounds, withered arms, a-v shunts
- Blood pressure cuff too small or too large to fit on arm
Right arm exclusion
- Blood has been drawn from right arm within the last week
- Presence of the following: rash, gauze dressing, cast, edema, paralysis, tubes, open sores or wounds, withered arm, a-v shunt
- Right mastectomy
- Right arm amputation
- Cast on right arm
Note: For respondents younger than 18, the anthropometric component must be completed prior to the completion of this component. If the Urine Component is not completed, the respondent should be encouraged to empty his bladder prior to the BP measurement.
Before taking the six measurements, the respondent will rest for a period of five minutes.
BPM_N101
Instruction: Record how the first set of data will be captured.
- Electronically
- Manually (Go to BPM_Q110)
Note: Don’t Know and Refused are not allowed. [All respondents]
Automated blood pressure measurement
BPM_Q101
Now I will take your blood pressure and heart rate using an automated blood pressure cuff. During this test you will need to sit with your feet flat on the floor with your back against the back rest of the chair, and have your right arm straight on the table.
Instruction: Select the appropriate cuff size based on arm circumference, secure it on the right arm and ensure the respondent is in the correct seated position.
Note: Don’t Know is not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Blood Pressure Component
If respondent refuses, go to BPM_Q110.
BPM_Q102
For respondents 14 or older:
The blood pressure cuff will inflate automatically once every minute, applying pressure to your arm. A total of six measures will be taken. I will stay in the room for the first measurement but will leave the room for all others. You should not move or talk during the test, and you need to keep both feet flat on the floor. It is important that you stay relaxed to ensure we get good results. Do you have any questions before we begin?
Instruction: Answer any questions as thoroughly as possible.
For respondents younger than 14:
The blood pressure cuff will fill with air, squeezing your arm a little. It will do this 6 times. During the test you cannot talk, and you need to sit really still and keep both feet flat on the floor or step. You should stay relaxed to ensure we get good results. Do you have any questions before we begin?
Instruction: Answer any questions as thoroughly as possible.
Note: Don’t Know and Refused are not allowed.
BPM_R103
Now I will start the machine.
Instruction: Press <Start> on the BPTru screen. Check that the BPTru collects the first measurement properly. Allow the BPTru to collect six measurements. Lock the fields containing the data from the BPTru. Save the measurements and go to BPM_N160.
Note: Don’t Know and Refused are not allowed.
Manual blood pressure measurement
BPM_Q110
Now I will take your blood pressure and heart rate. During this test you will need to sit with your feet flat on the floor with your back against the backrest of the chair, and have your right arm straight on the table with the palm facing up.
Instruction: Select the appropriate cuff size based on arm circumference, secure it on the right arm and ensure the respondent is in the correct seated position.
Determine the maximum inflation level.
Note: Don’t Know is not allowed.
All respondents except those meeting the exclusion criteria at the beginning of the Blood Pressure Component
If respondent refuses, go to BPM_END.
BPM_R110
For respondents 14 or older:
I will take your blood pressure 6 times, and will measure your heart rate using this heart rate monitor. Y ou should not move or talk during the test, and you need to keep both feet flat on the floor. It is important that you stay relaxed to ensure we get good results. Do you have any questions before we begin?
Instruction: Show the heart rate monitor to the respondent and help put it on. Answer any questions as thoroughly as possible.
For respondents younger than 14:
I will take your blood pressure 6 times, and will measure your heart rate using this heart rate monitor. During the test you need to sit really still, you cannot talk and you must keep your feet flat on the floor or step. You should stay relaxed to make sure that we get good results . Do you have any questions before we begin?
Instruction: Show the heart rate monitor to the respondent and help put it on. Answer any questions as thoroughly as possible.
Note: Don’t Know and Refused are not allowed.
BPM_B110
Record the blood pressure and heart rate 6 times.
BPR_N1A
Instruction: Record the systolic blood pressure measurement. (insert measurement between 30 and 300 mmHg)
BPR_N1B
Instruction: Record the diastolic blood pressure measurement. (insert measurement between 30 and 200 mmHg)
Note: Refused is not allowed.
BPR_N2
Instruction: Record the heart rate. (insert measurement between 30 and 200 beats per minute)
Note: Refused is not allowed.
BPR_N3
Instruction: Record the reason if the measurement could not be taken.
Mark all that apply.
5 Deflation too slow
6 Deflation too fast
20 Indeterminate systolic blood pressure
21 Indeterminate diastolic blood pressure
88 Other – Specify (insert answer to a maximum of 80 characters)
BPM_N160
Instruction: Check the blood pressure and heart rate data.
- Accept the measurements
- Redo the measurements (Go to BPM_R191)
Note: If there are large discrepancies in 3 or more of the measurements, or if the variation between any of the systolic or heart rate measurements exceeds prescribed limits, then redo the measurements.
Then the following variables are calculated:
- average systolic blood pressure
- average diastolic blood pressure
- average resting heart rate.
[All respondents]
BPM_R191
The entire measurement sequence is repeated, up to 2 times, using the following script: If it needs to be repeated because the blood pressure is too high:
Your [blood pressure/heart rate] today is a little elevated. This sometimes happens when people are anxious about the clinic tests. I will leave you to sit and relax for five minutes then I will come back and redo the measures.
If it needs to be repeated because of a BPTRU error or too much variability between measurements:
There were too many problems with that set of measurements, so we have to do the test again. I will retake your blood pressure and heart rate, but this time I will remain in the room to monitor the results. Now I will retake your blood pressure and heart rate.
Note: Don’t Know and Refused are not allowed.
BPM_D411
Blood pressure norms for respondents 18 or older are calculated. If measures fall within normal ranges, go to BPM_END, otherwise go to go to BPM_R411.
BPM_R411
Your average blood pressure today was [average systolic BP]/[average diastolic BP] mmHg. Based on a report by the Canadian Coalition for High Blood Pressure Prevention and Control, this means your blood pressure is [above the acceptable range/moderately high/high/very high].
Instruction: Answer any questions as thoroughly as possible.
Note: Don’t Know and Refused are not allowed.
BPM_D412
Blood pressure norms for respondents younger than 18 are calculated. If measures fall within normal ranges, go to BPM_END, otherwise go to go to BPM_R412.
BPM_R412
Your average blood pressure today was [average systolic BP]/[average diastolic BP] mmHg. Based on The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents, this means your blood pressure is [high/very high].
Instruction: Answer any questions as thoroughly as possible. If the blood pressure of the respondent was still high after three measurements, the respondent will receive a letter with his report of measurements that they should take to their doctor.
BPM_END
To be completed by all respondents except those meeting the exclusion criteria:
- Respondents who have hemophilia
- Respondents who have received chemotherapy within the last 4 weeks
- Respondents who have any of the following on both arms: rashes; gauze dressings; casts; edema; paralysis; tubes; open sores or wounds; withered arms or limbs missing; damaged; sclerosed or occluded veins; allergies to cleansing reagents; burned or scarred tissue; shunt or IV on both arms.
PHI_R01
Hi, my name is… Please have a seat on the bench because I need to ask you a few questions before we begin.
Note: Don’t Know and Refused are not allowed.
PHI_END
BDC_Q11
In the past 2 months, that is, from [date two months ago] to yesterday, did you receive a blood transfusion?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Phlebotomy Component
BDC_Q12
In the past 2 months, did you donate blood?
- Yes
- No (Go to BDC_Q21)
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Phlebotomy Component
BDC_B13
What was the date when you last donated blood?
Instruction: Enter the day. (insert respondent answer between 1 and 31)
Instruction: Select the month.
- January
- February
- March
- April
- May
- June
- July
- August
- September
- October
- November
- December
Instruction: Enter a four-digit year.
(insert respondent answer between 1925 and 2009)
All respondents, except those meeting the exclusion criteria at the beginning of the Phlebotomy Component
BDC_Q21
Now I am going to do the blood draw. Have you ever had blood taken?
Instruction: Explain the procedure to the respondent and try to alleviate any anxiety.
Refused (Go to BDC_END)
Note: Don’t Know is not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Phlebotomy Component
BDC_D21
Determine the blood collection tube labels needed. Print the blood collection tube labels. Attach each label to the appropriate blood collection tube.
BDC_N23
Instruction: Ensure the blood collection tubes are in the correct order. Record which of the required tubes of blood were collected. Mark all that apply.
Note: Don’t Know is not allowed.
BDC_N24
Instruction: Record whether the respondent was seated or supine during the blood draw.
- Seated
- Supine
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Phlebotomy Component
If respondent refused when first tube of blood was to be drawn, go to BDC_END. If all required tubes were collected, go to BDC_END.
BDC_N25
Instruction: Record the reason if all required tubes were not collected.
- Respondent refused
- Respondent fainted
- Unable to find vein
- Blood flow stopped
- Physical limitation
- Other – Specify (insert reason to a maximum of 80 characters)
Note: Don’t Know and Refused are not allowed.
Respondents who did not provide all required tubes of blood
BDC_END
To be completed by all respondents except those meeting the exclusion criteria:
- Respondents in a wheelchair.
AM_N11
Instruction: Record whether an activity monitor is available.
- Yes
- No (Go to AM_END)
Note: Don’t Know and Refused are not allowed.
AM_R11
As part of this survey we will be measuring the daily activity patterns of our participants over a 7 day period. To do this, we would like you to wear an activity monitor for the next 7 days.
An activity monitor is a battery-operated electronic device that is worn on a belt around the waist (over the right hip). The monitor records all daily activities as electronic signals, and it does not need to be turned on or off. In fact, as you can see, there are no external displays or buttons.
These activity monitors are not like the step counters you may have seen offered as promotional items on cereal boxes. Our activity monitors are much more sophisticated.
Instruction: Hold up the activity monitor (on the belt) for display.
Note: Don’t Know and Refused are not allowed.
AM_Q11
Would you be willing to wear an activity monitor for the next 7 days?
- Yes(Go to AM_R21)
- No
Note: Don’t Know and Refused are not allowed.
All respondents not in a wheelchair
AM_N12
Instruction: Record the reason why the respondent is not willing to wear an activity monitor for the next 7 days.
- Burden
- Invasive
- Aesthetics
- Away during the collection period
- Anticipating change in normal activity
- Sick or laid up
- Worried about losing or damaging the device
- Other – Specify (insert reason up to a maximum of 80 characters)
Go to AM_END.
Note: Don’t Know and Refused are not allowed.
AM_R21
You are to put the activity monitor on every day as soon as you wake up in the morning and wear it all day until you go to bed at night. You can wear the activity monitor either over or under your clothes, but you must make sure that it is positioned over your right hip, and that the belt is snug.
Instruction: Assist the respondent in putting the belt on. Check to ensure the belt fits snugly around the waist and that the activity monitor is positioned over the right hip. Ensure the monitor is positioned top up and is in line with the supraspinale.
Note: Don’t Know and Refused are not allowed.
AM_N21
Instruction: Record whether the respondent took an activity monitor.
- Yes (Go to AM_N31)
- No
Note: Don’t Know and Refused are not allowed.
AM_N22
Instruction: Record the reason why the respondent did not take an activity monitor.
- Burden
- Invasive
- Aesthetics
- Away during the collection period
- Anticipating change in normal activity
- Sick or laid up
- Worried about losing or damaging the device
- Other – Specify (insert reason up to a maximum of 80characters)
Go to AM_END.
Note: Don’t Know and Refused are not allowed.
AM_N31
Instruction: To log in the serial number of the activity monitor either read the number from the monitor case and manually type this number into the answer field or use the bar code wand to scan the bar code on the monitor case. (insert serial number between A000001 and Z999999)
Note: Don’t Know and Refused are not allowed.
AM_N32
Instruction: To log in the waybill number of the pre-paid envelope either read the number from the envelope and manually type this number into the answer field or use the bar code wand to scan the bar code on the envelope. (insert waybill number between AA000000001 and ZZ999999999CA)
Note: Don’t Know and Refused are not allowed.
AM_R33
On [date 8 days after clinic visit] we would like you to put the activity monitor and the belt into this pre-paid envelope. You should put this envelope into any Canada Post mailbox at your earliest convenience.
A full description of what the activity monitor is, what it measures, how it works, and why it is important is contained in the handouts in the mail-back envelope.
Instruction: Show the handouts to the respondent.
Note: Don’t Know and Refused are not allowed.
AM_END
To be completed by all respondents except those meeting the exclusion criteria:
- Respondent with a stoma
- Respondents with an acute respiratory condition such as cold, bronchitis, flu.
- Respondents with a significant language barrier.
- Women who are more than 27 weeks pregnant.
- Respondents who have suffered a heart attack within the last 3 months.
- Respondents who have had major surgery on chest or abdomen within the last 3 months.
- Respondents taking medication for tuberculosis.
- Respondents who have difficulty breathing at rest.
- Respondents who have a persistent cough.
SPR_R11
First I need to ask a couple of health-related questions to make sure we are able to do the lung function test for you today.
SPR_Q11
Have you had a heart attack within the past 3 months?
- Yes (Go to SPM_END)
- No
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Spirometry Component
SPR_Q12
Have you had major surgery on your chest or abdomen in the past 3 months?
- Yes (Go to SPM_END)
- No
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Spirometry Component
SPR_END
SPM_Q021
For respondents 12 or older:
Now I’d like to measure your lung function using a basic breathing test that greatly depends on effort.
Instruction: Demonstrate the test (without using the mouthpiece).
For respondents younger than 12:
Now I would like to test your lungs to see how well they work.
Instruction: Demonstrate the test (without using the mouthpiece).
Note: Don’t Know is not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Spirometry Component [SPR_Q11 = 2 and SPR_Q12 = 2]
SPM_N022
Instruction: Record the appropriate race adjustment for the respondent.
- White
- Black
- Hispanic
- Asian
- Other
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Spirometry Component [SPR_Q11 = 2 and SPR_Q12 = 2]
SPM_N023
Instruction: Check the data to be sent to the spirometer.
- Transmit the data
Note: Don’t Know and Refused are not allowed.
SPM_R024
Instruction: Open the KoKo software. Follow the instructions on importing and retrieving respondent information. In the KoKo patient information screen, chose which set of predicted norms is to be applied:
If respondent is younger than 8 use:
“Corey 1976”
If respondent is 8 years or older use:
“Hankinson (NHANES III)”
Note: Don’t Know and Refused are not allowed.
Once the predicted have been chosen, an Ethnic Group must be chosen (see Appendix IV).
SPM_Q031
For respondents 12 or older:
During the test you will need to wear a nose clip to ensure that no air escapes from your nose. You should sit up straight throughout the test, with both feet flat on the floor. Before we start the test you will put the mouthpiece in your mouth, forming a good seal, with your lips and teeth on the outside of the mouthpiece so that air cannot escape. You will then be asked to take a few normal breaths. At the end of the last normal breath, you should take a big breath in, filling your lungs with as much air as possible. Then you will immediately blast all the air out as forcefully and as quickly as you can. Do not hold your breath before blowing out. Keep blowing out until you have absolutely no air left in your lungs. You may believe there is no more air in your lungs but you must try to keep blowing out for at least 6 seconds. I will be encouraging you to keep blowing, and I will tell you when to stop. When I do, take a big breath in once again.
Respondents aged 12 and over, except those meeting the exclusion criteria at the beginning of the Spirometry Component [Respondents aged 12 and over who answered SPR_Q11 = 2 and SPR_Q12 = 2]
Instruction: Demonstrate the test (without using the mouthpiece).
For respondents younger than 12:
During the test I will have you wear a nose clip so that you breathe only through your mouth. You should sit up straight and keep both feet flat on the floor or the stool. Before we start the test you will put the mouthpiece in your mouth, with your lips and teeth on the outside of the mouthpiece, making sure that no air can escape. I will then ask you to take a few normal breaths. At the end of the last normal breath, you should take a big breath in, breathing in as much air as you can. Then you will blast out all the air as hard and as fast as possible. Do not hold your breath before blowing out. Keep blowing out until you have absolutely no air left in your lungs. You may believe there is no more air in your lungs but you must try to keep blowing out for at least 6 seconds. I will be encouraging you to keep blowing, and I will tell you when to stop. When I do, take a big breath in once again.
Instruction: Demonstrate the test (without using the mouthpiece).
Note: Don’t Know is not allowed.
Respondents aged 11 and under, except those meeting the exclusion criteria at the beginning of the Spirometry Component [ [Respondents aged 11 and under who answered SPR_Q11 = 2 and SPR_Q12 = 2]
SPM_Q032
For respondents 12 or older:
I will be giving verbal encouragement throughout the test. To get the best possible result, you really must provide a maximal effort. I need 3 good tests to record your scores but we may do as many as 8 tests to ensure we have the best tests recorded.
Do you have any questions before we begin?
Respondents aged 12 and over, except those meeting the exclusion criteria at the beginning of the Spirometry Component [Respondents aged 12 and over who answered SPR_Q11 = 2 and SPR_Q12 = 2]
For respondents younger than 12:
I will be talking to you during the test to remind you of what you are supposed to do. To make sure we get the best result, you must try to blow as hard as you can. I need you to do at least 3 good tests to record your scores but we may do as many as 8 tests to ensure we have the best one.
Do you have any questions before we begin?
Note: Don’t Know is not allowed.
Respondents aged 11 and under, except those meeting the exclusion criteria at the beginning of the Spirometry Component [ [Respondents aged 11 and under who answered SPR_Q11 = 2 and SPR_Q12 = 2]
SPM_R100
Instruction: Ensure the spirometry test results have been saved in the KOKO folder
Note: Don’t Know and Refused are not allowed.
SPM_N100
Instruction: Press <1> to save the measurements in Blaise.
- Save the measurements
Note: Don’t Know and Refused are not allowed.
If there are less than 3 trials performed, go to SPM_N901. Otherwise, If SPM_N101 = Empty or SPM_N901 = RESPONSE, go to SPM_END.
SPM_N901
Instruction: Why were fewer than 3 trials performed?
- Respondent unable to continue for health reasons
- Respondent unable to understand technique
- Respondent refuses to continue
- Equipment problem
- Other – Specify (insert reason to a maximum of 80 characters)
Note: Don’t Know and Refused are not allowed.
If no trials were performed, or if SPM_N901 = RESPONSE, go to SPM_END.
Otherwise, the following variables are calculated:
- percent predicted Forced Vital Capacity (FVC)
- percent predicted Forced Expiratory Volume (FEV1).
SPM_END
To be completed by all respondents except those meeting the exclusion criteria:
- Respondents who gave a positive response to PAR-Q questions 1, 2, 3 or 6 (automatic) or 4, 5 or 7 (depending upon probing). See PAR-Q in Appendix III.
- Respondents taking heart rate or blood pressure medications.
- Women who are more than 12 weeks pregnant.
- Respondents with resting heart rate ≥ 100 bpm or resting blood pressure > 144/94 mm Hg as determined during the Blood Pressure component.
- Mentally and physically impaired individuals, at the discretion of the Health Measures Specialist. Every effort should be made to be inclusive of individuals with disabilities provided that all safety precautions are taken.
- Respondents who have difficulty breathing at rest.
- Respondents taking medication for a breathing condition that worsens during exercise, but do not have their medication with them (as assessed during the Screening Component).
- Respondents who have given a blood donation in the past 24 hours.
- Respondents who appear ill or complains of fever.
- Respondents who have a persistent cough.
- Respondents who have lower extremity swelling.
- Respondents with an insulin pump.
- Respondents with a colostomy bag.
- Respondents who are 70 or older.
- Respondents who have opted for a home visit.
The starting stage and ceiling heart rate are calculated, based on the respondent’s age and sex, and are displayed on the screen. For example,
- Starting stage : 2
- Ceiling heart rate : 152 bpm
AFT_R10
The next test we are going to do is a stepping test to measure your fitness level. The test will require you to step up and down this set of stairs continuously to music for 3 minutes at a time. In total there are 8, 3-minute stages. You are starting at stage [1 to 8]. During the test you will wear a heart rate monitor so that I can watch your heart rate. At the end of each 3 minute stepping stage you will be asked to stop exercising. Stop where you are and I will check your heart rate to see if you should do another stage. You will continue going through the stages until your heart rate meets a ceiling value for your age and sex. Your ceiling heart rate is
[ceiling heart rate in bpm]. If your heart rate is at or above this number then I will stop the test. At the end of the test you will slowly walk around for 2 minutes. Then you will sit down and I will take your blood pressure and heart rate a few more times to make sure that you are recovering well from the test.
Instruction: Show the heart rate monitor to the respondent and help to put it on.
Note: Don’t Know and Refused are not allowed.
AFT_R11
For respondents 14 and older:
During the test you need to go up and down the stairs following the beat of the music. The stepping pattern goes like this, “step, step, up, step, step, down”. When you are stepping you should never have both feet on the first step at the same time, and you need to make sure that both feet are placed fully on the top step. If you reach the final 2 stepping stages the stepping pattern will change to a single "step up, step down" pattern. I will play the music and show you how the test is done. Do you have any questions?
For respondents younger than 14:
During the test you need to go up and down the stairs following the beat of the music. The stepping pattern goes like this, “step, step, up, step, step, down”. When you are stepping you should never have both feet on the first step at the same time, and you need to make sure that both feet are placed fully on the top step. I will play the music and show you how the test is done. Do you have any questions?
Instruction: Play the music and demonstrate the stepping pattern at respondent’s starting stage.
Note: Don’t Know and Refused are not allowed.
AFT_N11
Instruction: Record the heart rate. (insert measurement between 30 and 200 beats per minute)
Note: Record heart rate at the end of each stage up to stage 8. If the ceiling heart rate is reached at any time, stop the test and go to AFT_R21.
All respondents, except those meeting the exclusion criteria at the beginning of the mCAFT Component
AFT_R21
The test is finished. I would like you to slowly walk around for 2 minutes and then I will have you sit down so that I can take your blood pressure and heart rate again.
Note: Don’t Know and Refused are not allowed.
AFT_N22
Instruction: Record how the data will be captured.
- Electronically
- Manually (Go to AFT_Q31)
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the mCAFT Component
Automated blood pressure measurement
AFT_Q30
Now I will take the first of two post exercise blood pressure and heart rate measurements using this automated blood pressure cuff. During this test you will need to sit with your feet flat on the floor with your back against the back rest of the chair, and have your right arm straight on the table. You should not move or talk during the measurement.
Instruction: Select the appropriate cuff size based on arm circumference, secure it on the right arm and ensure the respondent is in the correct seated position. Set the BPTru to collect a single measure (set cycle to SP). Start the BPTru 2 minutes after the respondent has completed the mCAFT. Save the measurements and go to AFT_Q40
Note: Refused (Go to AFT_Q31)
Note: Don’t Know is not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the mCAFT Component [AFT_N22 = 1 or AFT_Q30 = RF]
Manual blood pressure measurement
AFT_Q31
Now I will take the first of two post exercise blood pressure and heart rate measurements. During this test you will need to sit with your feet flat on the floor with your back against the back rest of the chair, and have your right arm straight on the table with the palm facing up. You should not move or talk during the measurement.
Instruction: Select the appropriate cuff size based on arm circumference, secure it on the right arm and ensure the respondent is in the correct seated position. Determine the maximum inflation level.
Note: Don’t Know is not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the mCAFT Component [AFT_N22 = 2 or AFT_Q30 = RF]
BPR_N1A
Instruction: Record the systolic blood pressure measurement. (insert measurement between 30 and 300 mmHg)
BPR_N1B
Instruction: Record the diastolic blood pressure measurement. (insert measurement between 30 and 200 mmHg)
BPR_N2
Instruction: Record the heart rate. (insert measurement between 30 and 200 beats per minute)
BPR_N3
Instruction: Record the reason if the measurement could not be taken.
Mark all that apply.
5 Deflation too slow
6 Deflation too fast
20 Indeterminate systolic blood pressure
21 Indeterminate diastolic blood pressure
88 Other – Specify (insert measurement to a maximum of 80 characters)
AFT_Q40
I will now take a second blood pressure and heart rate measurement.
Instruction: Begin the measurement 3.5 minutes after the respondent has completed the mCAFT.
Note: Second and subsequent measurements are captured manually or electronically following the same procedures as were completed for the first measurement.
All respondents except those meeting the exclusion criteria at the beginning of the mCAFT Component [AFT_N22 = 1, excluding refusals to AFT_Q30]
If [average systolic blood pressure] < 145 and [average diastolic blood pressure] < 95 and [resting heart rate] < 100, go to AFT_END.
AFT_R49
The entire measurement sequence is repeated up to two more times, at 6 minutes and 8 minutes after the respondent has completed the mCAFT, using the following script:
Your [blood pressure and heart rate are/blood pressure is/heart rate is] still high from doing the exercise so please sit and relax for 2 minutes and then I will take your blood pressure and heart rate again .
Then the following variables are calculated:
- oxygen cost
- aerobic fitness score
- aerobic fitness norms for respondents aged 15 to 69
- aerobic fitness norms for respondents younger than 15.
Note: Don’t Know is not allowed.
If the heart rate at any of the 8 stages was recorded as “Don’t Know” go to AFT_N81.
Otherwise, go to AFT_END.
AFT_N81
Instruction: Record the reason why the respondent did not complete the test.
- Refusal
- Unable to maintain proper cadence
- Dizziness
- Extreme leg pain
- Nausea
- Chest pain
- Facial pallor
- Other – Specify (insert reason to a maximum of 80 characters)
Note: Don’t Know and Refused are not allowed.
Respondents who did not complete all required stages
AFT_END
To be completed by all respondents except those meeting the exclusion criteria:
- Respondent gave a positive response(s) to PAR-Q questions 5, 6 or 7 (depending upon probing). See the PAR_Q in Appendix III.
GSI_R1
Next I am going to measure your upper body strength with a hand grip dynamometer. You will perform this test two times on each hand, alternating hands each time. When performing the test you hold your hand away from your body, and squeeze the handle as hard as you can, blowing out while you squeeze.
Instruction: Demonstrate the procedure while explaining the technique.
Note: Don’t Know and Refused are not allowed.
GSI_R2
Hold the handle so that the 2nd joints of your fingers fit snugly under the handle; we can adjust the size if necessary. Remember, hold your arm straight and away from your body, and squeeze the handle as hard as you can, blowing out while you squeeze.
Note: Don’t Know and Refused are not allowed.
GSI_END
GSM_N11
Instruction: Record the first grip strength measurement for the right hand. (insert measurement between 0 and 120 kilograms of pressure)
All respondents, except those meeting the exclusion criteria at the beginning of the Grip Strength Component
GSM_S11
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
GSM_N12
Instruction: Record the first grip strength measurement for the left hand. (insert measurement between 0 and 120 kilograms of pressure)
All respondents, except those meeting the exclusion criteria at the beginning of the Grip Strength Component
GSM_S12
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
GSM_N21
Instruction: Record the second grip strength measurement for the right hand. (insert measurement between 0 and 120 kilograms of pressure)
All respondents, except those meeting the exclusion criteria at the beginning of the Grip Strength Component
GSM_S21
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
GSM_N22
Instruction: Record the second grip strength measurement for the left hand. (insert measurement between 0 and 120 kilograms of pressure)
All respondents, except those meeting the exclusion criteria at the beginning of the Grip Strength Component
GSM_S22
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
Then the following variables are calculated:
- total hand grip strength
- grip strength norms for respondents aged15 to 69
- grip strength norms for respondents younger than 15.
GSM_END
To be completed by all respondents except those meeting the exclusion criteria:
- Respondents who gave positive response(s) to PAR-Q questions 5 and 7 (depending upon probing). See the PAR-Q in Appendix III.
- Women who are more than 12 weeks pregnant.
- Respondents who are 70 or older.
- Respondents who have a colostomy bag.
- Respondents who opted for a home visit.
SRI_R1
The next test we’re going to do is called a sit-and-reach test, which will measure your back and hamstring flexibility. Before we start the test, we will do some stretches to loosen your leg muscles. I will do the stretches with you to show you how they are done. Sit on the floor with one leg out straight and the bottom of your other foot tucked into the straight leg. Reach forward towards the toe of your straight leg only until you feel a slight stretch in the back of your leg. You should not feel pain and you should not bounce. We will hold the stretch for 20 seconds and then we will switch to the other leg. We will do the stretch twice on each leg.
Instruction: Have the respondent sit on the mat in the modified hurdle stretch position. Do the stretch twice on each leg, holding the stretch for 20 seconds each time. Perform the stretches alongside the respondent.
Note: Don’t Know and Refused are not allowed.
SRI_R2
Before you do the test you will need to remove your shoes. I will demonstrate how to do it. When doing the test:
- Sit with your legs out straight in front of you with your feet flat against the board and your legs about 6 inches or 15 cm apart. You must not bend your knees
- Put your arms straight out in front of you and put your hands on top of one another
- Reach forward pushing the sliding marker along the scale with your fingertips as far as possible. Do not bounce
- When you are reaching forward you should breathe out and lower your head to help you reach farther
- When you have reached as far as you can you must hold your reach for 2 seconds. I will count this aloud for you and tell you when to sit up again
Instruction: Demonstrate the movement while explaining the main points of the test.
Note: Don’t Know and Refused are not allowed.
SRI_R3
Do you have any questions before we begin?
Instruction: Answer any questions as thoroughly as possible.
Note: Don’t Know and Refused are not allowed.
SRI_END
SRM_N01
Instruction: Record the first sit and reach attempt.
(insert measurement between 0.0 and 75.0 centimetres)
All respondents, except those meeting the exclusion criteria at the beginning of the Sit and Reach Component
SRM_S01
Instruction: If the measurement could not be taken, specify the reason.
Note: Don’t Know, Refused and Empty are not allowed.
SRM_N02
Instruction: Record the second sit and reach attempt.
(insert measurement between 0.0 and 75.0 centimetres)
All respondents, except those meeting the exclusion criteria at the beginning of the Sit and Reach Component
SRM_S02
Instruction: If the measurement could not be taken, specify the reason.
Note: Don’t Know, Refused and Empty are not allowed.
Then the following variables are calculated:
- sit and reach measure
- sit and reach norms for respondents aged 15 to 69
- sit and reach norms for respondents younger than 15.
SRM_END
To be completed by all respondents except those meeting the exclusion criteria:
- Positive response(s) to PAR-Q questions 1, 2, 3 (automatic) and 5, 6 and 7 (depending upon probing). See the PAR-Q in Appendix III.
- Women who are more than 12 weeks pregnant.
- Respondents who are 70 or older.
- Respondents with resting heart rate > 100 bpm or blood pressure > 144/94 mmHg as determined during the screening component.
- Mentally and physically disabled individuals (at the discretion of the HMS).
- Respondents who have difficulty breathing at rest.
- Respondents with a persistent cough.
- Respondent with lower extremity swelling.
- Respondents who appears ill or complains of fever.
- Respondents with a colostomy bag.
- Respondents who opted for a home visit.
PCI_R1
The next test we’re going to do is called partial curl-ups, which are similar to sit-ups or crunches. I will demonstrate how to do them correctly and then I will have you try them.
Instruction: Demonstrate a proper curl-up, and state:
- When curling up, your hands should slide along the surface of the mat and your fingertips must touch the far edge of the metal strap
- When curling down, your head must return to the mat
- You need to curl up on a beep and down on a beep, following the metronome
- You should breathe out when curling up and in when curling down
- Your heels must stay in contact with the mat or floor at all times
- Only good repetitions will be counted to a maximum of 25 (i.e., 1 minute at 50 bpm)
- I will correct your form, but after two consecutive bad repetitions the test will be stopped.
Note: The bad repetitions need to be the same (e.g., rep.1=head does not touch the floor, rep 2=head does not touch the floor)
Note: Don’t Know and Refused are not allowed.
PCI_R2
Now I will have you lie on your back on the mat. Bend your legs to 90 degrees with your legs shoulder width apart. Keep your heels in contact with the mat or floor. I will make sure your legs are at 90 degrees before we start. Place your arms straight by your sides so that your fingertips are touching the edge of the metal strap.
Instruction: Help the respondent get into position. Use the goniometer to ensure leg angle is 90 degrees. Adjust the metal strap to meet the respondent’s fingertips.
Note: Don’t Know and Refused are not allowed.
PCI_R3
Remember, in order for a partial curl-up to be counted you must keep the correct form and timing. When doing the test, I will correct you if you do an incorrect curl-up and will allow you to continue if you can. If you are unable to correct your form we will stop the test. A maximum of 25 curl-ups will be done. Do you have any questions?
Instruction: Answer any questions as thoroughly as possible.
Note: Don’t Know and Refused are not allowed.
PCI_R4
I will play the metronome now so that you can listen to the beat. When you are ready you can begin the test.
Note: Don’t Know and Refused are not allowed.
PCI_END
PCM_N01
Instruction: Record the total number of partial curl-ups completed in one minute.
(insert measurement between 0 and 25)
All respondents, except those meeting the exclusion criteria at the beginning of the Partial Curl-Up Component
PCM_S01
Instruction: If the measurement could not be taken, specify the reason.
(insert respondent answer to a maximum of 80 characters)
Note: Don’t Know, Refused and Empty are not allowed.
PCM_D11
The partial curl-up norms for respondents aged 15 to 69 are calculated.
PCM_END
To be completed by all respondents.
OHI_R01
Hello, my name is … and I will be recording the results of your dental examination on this computer, and this is …, a licensed dentist who will be doing your dental exam today. Please sit back in this chair, relax, and make yourself as comfortable as possible.
Note: Don’t Know and Refused are not allowed.
OHI_END
OHQ_R11
First, I have a few questions about the health of your teeth.
Note: Don’t Know and Refused are not allowed.
OHQ_Q11
Do you think you have any untreated dental conditions?
- Yes
- No (Go to OHQ_Q21)
Don’t Know, Refused (Go to OHQ_Q21)
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_Q12
What untreated dental condition(s) do you think you have?
Instruction: Mark all that apply.
- Prevention
- Fillings
- Temporomandibular joint disorder (TMD)
- Surgery
- Periodontics
- Esthetics
- Endodontics
- Orthodontics
- Soft tissue
- Prosthetics – partial or full denture
- Prosthetics – implant, bridge or crown
- Other – Specify (insert condition to a maximum of 80 characters)
Respondents believing they have an untreated dental condition [OHQ_Q11 = 1]
OHQ_Q21
In the past month, that is, from [date last month] to yesterday, have you had a toothache?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_Q22
In the past month, have you had pain in your teeth when consuming hot or cold foods or drinks?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_Q23
In the past month, have you had:
… severe tooth or mouth pain at night?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_Q24
In the past month, have you had:
… pain in or around your jaw joints?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_Q25
In the past month, have you had:
… other pain in your mouth?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_Q26
In the past month, have you had bleeding gums when brushing your teeth?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_Q27
In the past month, have you had:
… persistent dry mouth?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_Q28
In the past month, have you had:
… persistent bad breath?
- Yes
- No
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHQ_END
If respondent has hemophilia or has received chemotherapy in the last four weeks, go to OHR_END.
OHR_R11
Next I need to ask a few health-related questions to make sure we are able to do the complete dental examination for you.
Note: Don’t Know and Refused are not allowed.
OHR_Q11
Do you have to take antibiotics (for example, penicillin) before you have a check-up or get dental care?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed.
Respondents who do not have hemophilia or have not received chemotherapy in the previous 4 weeks [PHC_Q51 = 1 or PHC_Q52 = 1]
OHR_Q12
Have you ever been diagnosed by a health professional with a heart murmur that requires you to take antibiotics for dental treatment?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed.
Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q11 = 2]
OHR_Q13
Have you ever been diagnosed by a health professional with a heart valve problem?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q12 = 2]
OHR_Q14
Have you ever been diagnosed by a health professional with:
… congenital heart disease?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q13 = 2]
OHR_Q15
Have you ever been diagnosed by a health professional with:
… bacterial endocarditis?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q14 = 2]
OHR_Q16
Have you ever been diagnosed by a health professional with:
… rheumatic fever?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q15 = 2]
OHR_Q17
Have you had bypass surgery in the past year?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q16 = 2]
OHR_Q18
Do you have a pacemaker or other automatic defibrillator?
- Yes (Go to OHR_Q19)
- No (Go to OHR_Q20)
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q17 = 2]
OHR_Q19
Have you had your pacemaker or other automatic defibrillator for less than one year?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who have a pacemaker or other automatic defibrillator [OHR_Q18 = 1]
OHR_Q20
Do you have other artificial material in your heart, veins or arteries?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q18 = 2 or OHR_Q19 = 2]
OHR_Q21
Have you ever had a joint replacement?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q20 = 2]
OHR_Q22
Have you ever received an organ transplant?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q21 = 2]
OHR_Q23
Do you have kidney disease that requires dialysis?
- Yes (Go to OHR_D25)
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q22 = 2]
OHR_Q24
Are you immuno-supressed or are you on immuno-suppression therapy? (For example, chemotherapy.)
- Yes
- No
Note: Don’t Know and Refused are not allowed. Respondents who do not have hemophilia, have not received chemotherapy in the previous 4 weeks, and have answered "no" to all previous questions in the Oral Health Restrictions (OHR) block [OHR_Q23 = 2]
OHR_D25
If respondent answered yes to any of the Oral Health Restriction questions, probing will not be performed.
OHR_END
The probing portion of the oral health exam is to be completed by all respondents except those meeting the exclusion criteria:
- Respondents with hemophilia
- Respondents who have had chemotherapy within the past 4 weeks
- Respondents who answer “yes” to any question in the Oral Health Restrictions block (OHR)
- Respondents who are younger than 15
OHE_R11
Now I’m going to do a simple dental examination. The only instruments I will use to look at your mouth and teeth are a hand mirror and these explorers. You should not feel any pain and no x-rays will be taken. I just want to get a sense of the health of your teeth and mouth.
Instruction: Show the instruments to the respondent. If necessary, demonstrate the explorers on the respondent’s fingernail.
Note: Don’t Know and Refused are not allowed.
OHE_N11
Instruction: Record the dental status of the respondent.
- Dentate – both arches
- Dentate – upper arch only
- Dentate – lower arch only
- Edentulous with one or more implants
- Edentulous
Note: Don’t Know is not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
If respondent refuses, go to OHE_END.
OHE_N12
Instruction: Record the prosthetic status of the upper arch of the respondent.
Mark all that apply.
- No prosthetics
- Fixed bridge
- Implant
- Partial denture – acrylic
- Partial denture – cast chrome
- Full denture
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHE_N13
Instruction: Record the prosthetic status of the lower arch of the respondent.
Mark all that apply.
- No prosthetics
- Fixed bridge
- Implant
- Partial denture – acrylic
- Partial denture – cast chrome
- Full denture
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHE_N14
Instruction: Record the mucosal status of the respondent.
Mark all that apply.
- No mucosal abnormalities
- Angular chelitis
- Mucosal white patches
- Denture stomatitis
- Denture induced hyperplasia (epulis)
- Glossitis
- Sinus or fistula
- Aphthous ulcer
- Traumatic or unspecified ulcer
- Other - Specify (insert status to a maximum of 80 characters)
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
If OHE_N14 = 3, go to OHE_N15. If OHE_N11 = 4 or 5, go to OHE_N51. If CON_AGE > 12, go to OHE_N21.
OHE_N15
Instruction: Record the type of mucosal white patches.
- Leukoplakia
- Lichen planus
- Candidiasis
Note: Don’t Know and Refused are not allowed. [OHE_N14C = 1]
If OHE_N11 = 4 or 5, go to OHE_N51. If respondent is older than 12, go to OHE_N21.
OHE_N20
Instruction: Record the fluorosis score for the most affected pair of teeth for teeth 12, 11, 21 or 22. If the two teeth are not equally affected, record the score for the less affected of the two.
- Normal
- Questionable
- Very mild
- Mild
- Moderate
- Severe
- All 4 anterior teeth absent
[Respondents aged less than 12 who answered OHE_N11 = (1, 2, 3)]
If OHE_N11 > 1, OHE_N12 = 6 (Upper arch full denture) and OHE_N13 = 6 (Lower arch full denture), go to OHE_N23.
OHE_N21
Instruction: Record all occlusal conditions that are present.
Mark all that apply.
- Acceptable occlusion
- Anterior crossbite
- Severe crowding
- Severe spacing
- Posterior crossbite
- Anterior open bite (> 1 mm)
- Excessive overbite (100% or more)
- Excessive overjet (> 9 mm)
- Midline shift (> 4 mm)
Note: Refused is not allowed. [OHE_N11 = 1]
If OHE_N12 = 6 (Upper arch full denture) and OHE_N13 = 6 (Lower arch full denture), go to OHE_N23.
OHE_N22
Instruction: Record the current orthodontic treatment status of the respondent.
- No orthodontic treatment
- Removable appliances
- Fixed appliances
- Both fixed and removable appliances
- Retainer – post completion
Note: Don’t Know and Refused are not allowed. [OHE_N11 = (1, 2, 3)]
If OHE_N22 > 1, go to OHE_N31. If OHE_N12 = 6 (Upper arch full denture) and OHE_N13 = 6 (Lower arch full denture), go to OHE_N51.
OHE_N23
Instruction: Record whether the respondent has received orthodontic treatment in the past.
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N11 = (1, 2, 3) and OHE_N22 = 1]
If OHE_N12 = 6 (Upper arch full denture) and OHE_N13 = 6 (Lower arch full denture), go to OHE_N51.
OHE_N31
Instruction: Record the worst score for each tooth.
Gingivitis:
- No inflammation
- Mild inflammation
- Moderate inflammation
- Severe inflammation
- Tooth missing
Teeth Recorded:
Tooth 16 (55)
Tooth 12 (52)
Tooth 24 (64)
Tooth 36 (75)
Tooth 32 (72)
Tooth 44 (84)
Note: Teeth numbered in brackets indicate primary (baby) teeth and all other teeth numbers indicate permanent teeth.
Note: Don’t Know and Refused are not allowed. [OHE_N11 = (1, 2)]
OHE_N32
Instruction: Record the worst score for each condition for each sextant (by tooth or pair of teeth).
Debris:
- No soft debris or stain
- Less than 1/3 of surface covered
- 1/3 to 2/3 of surface covered
- More than 2/3 of surface covered
- Teeth missing
Calculus:
- No calculus
- Less than 1/3 of surface covered
- 1/3 to 2/3 of surface covered
- More than 2/3 of surface covered
Attachment loss: (insert distance in millimetres between 0 and 12)
Probing score: (insert depth in millimetres between 0 and 9)
Teeth Recorded:
Teeth 17 & 16 (55)
Tooth 11 (51)
Teeth 26 & 27 (65)
Teeth 37 & 36 (75)
Tooth 31 (71)
Teeth 46 & 47 (85)
Note: Don’t Know is not allowed, except for Attachment loss and Probing score.
Note: Refused is not allowed, expect for Probing score.
OHE_N41
For baby teeth, display the following list of categories: Instruction: Record the condition of each tooth in the appropriate box.
1. Sound – never decayed or restored
2. Sound – crown sealed, never decayed or otherwise restored
3. Missing – due to orthodontic treatment
4. Missing – due to trauma
5. Missing – due to caries or periodontal disease
6. Unerupted tooth, congenitally missing or unexposed root
7. Decayed severely
8. Decayed – pit and fissure caries
9. Decayed – smooth surface caries
10. Decayed – both smooth surface and pit and fissure caries
12. Filled with amalgam, no other decay
13. Filled with other material (resin, GIC, inlay, crown), no other decay
14. Filled with amalgam and other material (resin, GIC, inlay, crown), no other decay
15. Filled with amalgam, no other decay, but filling is defective and needs replacement
16. Filled with other material (resin, GIC, inlay, crown) but filling is defective and needs replacement
17. Filled with amalgam and other material (resin, GIC, inlay, crown) but filling is defective and needs replacement
20. Fractured due to trauma
21. Other
OHE_N41
For crowns of adult teeth, display the following list of categories: Instruction: Record the condition of each tooth in the appropriate box.
1. Sound – never decayed or restored
2. Sound – crown sealed, never decayed or otherwise restored
3. Missing – due to orthodontic treatment
4. Missing – due to trauma
5. Missing – due to caries or periodontal disease
6. Unerupted tooth, congenitally missing or unexposed root
7. Decayed severely
8. Decayed – pit and fissure caries
9. Decayed – smooth surface caries
10. Decayed – both smooth surface and pit and fissure caries
12. Filled with amalgam, no other decay
13. Filled with other material (resin, GIC, inlay, crown), no other decay
14. Filled with amalgam and other material (resin, GIC, inlay, crown), no other decay
15. Filled with amalgam, no other decay, but filling is defective and needs replacement
16. Filled with other material (resin, GIC, inlay, crown) but filling is defective and needs replacement
17. Filled with amalgam and other material (resin, GIC, inlay, crown) but filling is defective and needs replacement
18. Bridge abutment, special crown or veneer
19. Implant
20. Fractured due to trauma
21. Other
OHE_N41
For roots of adult teeth, display the following list of categories. Data entry for respondents younger than 18 is not possible.
Instruction: Record the condition of each tooth in the appropriate box.
1. Sound – never decayed or restored
3. Missing – due to orthodontic treatment
4. Missing – due to trauma
5. Missing – due to caries or periodontal disease
6. Unerupted tooth, congenitally missing or unexposed root
7. Decayed severely
11. Decayed – smooth surface caries
12. Filled with amalgam, no other decay
13. Filled with other material (resin, GIC, inlay, crown), no other decay
14. Filled with amalgam and other material (resin, GIC, inlay, crown), no other decay
15. Filled with amalgam, no other decay, but filling is defective and needs replacement
16. Filled with other material (resin, GIC, inlay, crown) but filling is defective and needs replacement
17. Filled with amalgam and other material (resin, GIC, inlay, crown) but filling is defective and needs replacement
19. Implant
20. Fractured due to trauma
21. Other
Note: Data are recorded for each tooth whether or not present.
OHE_N42
Instruction: Count and record the number of tooth surfaces with amalgam fillings. (insert measurement between 0 and 95)
Note: Don’t Know, Refused and Empty are not allowed. [OHE_N11 = (1, 2, 3)]
OHE_N43
Instruction: Record the condition of each tooth in the appropriate box.
- No evidence of traumatic injury
- Unrestored enamel fracture – does not involve dentin
- Unrestored enamel fracture – involves dentin
- Untreated damage – dark discolouration, swelling, fistula
- Restored fracture – full crown
- Restored fracture – other restoration
- Lingual restoration plus history of root canal treatment
- Other
Teeth Recorded :
Teeth 12 & 11
Teeth 21 & 22
Teeth 32 & 31
Teeth 41 & 42
Note: Don’t Know and Refused are not allowed.
OHE_N51
Instruction: Record the prosthetic needs of the upper arch of the respondent.
Mark all that apply.
- No prosthetics needed
- Fixed bridge
- Implant
- Denture repair or reline
- New partial denture
- New full denture
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHE_N52
Instruction: Record the prosthetic status of the lower arch of the respondent.
Mark all that apply.
- No prosthetics needed
- Fixed bridge
- Implant
- Denture repair or reline
- New partial denture
- New full denture
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHE_N53
Instruction: Record the treatment currently needed by the respondent.
Mark all that apply.
- No treatment needed
- Prevention
- Fillings
- Temporomandibular joint disorder (TMD)
- Surgery
- Periodontics
- Esthetics
- Endodontics
- Orthodontics
- Soft tissue
- Other – Specify (insert treatment to a maximum of 80 characters)
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHE_R60
We have now completed the examination. Thank you for your participation.
If OHE_N53 = 1, go to OHE_N71. If OHE_N53 = 3, go to OHE_N61. If OHE_N53 = 4, go to OHE_N62. If OHE_N53 = 5, go to OHE_N63. If OHE_N53 = 6, go to OHE_N64. If OHE_N53 = 8, go to OHE_N65. If OHE_N53 = 9, go to OHE_N66. If OHE_N53 = 10, go to OHE_N67. If OHE_N53 = 11, go to OHE_N68. Otherwise go to OHE_N71.
OHE_N61
Instruction: Record whether the respondent needs fillings urgently (i.e., within a week).
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N53 = 3]
OHE_N62
Instruction: Record whether the respondent needs treatment for Temporomandibular joint disorder (TMD) urgently (i.e., within a week).
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N53 = 4]
OHE_N63
Instruction: Record whether the respondent needs surgery urgently (i.e., within a week).
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N53 = 5]
OHE_N64
Instruction: Record whether the respondent needs periodontics urgently (i.e., within a week).
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N53 = 6]
OHE_N65
Instruction: Record whether the respondent needs endodontics urgently (i.e., within a week).
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N53 = 8]
OHE_N66
Instruction: Record whether the respondent needs orthodontics urgently (i.e., within a week).
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N53 = 9]
OHE_N67
Instruction: Record whether the respondent needs soft tissue treatment urgently (i.e., within a week).
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N53 = 10]
OHE_N68
Instruction: Record whether the respondent needs other treatment urgently (i.e., within a week).
- Yes
- No
Note: Don’t Know and Refused are not allowed.
[OHE_N53 = 11]
OHE_N71
Instruction: Was a serious medical condition that requires immediate attention discovered during the dental examination?
- Yes
- No (Go to OHE_END)
Note: Don’t Know and Refused are not allowed.
All respondents, except those meeting the exclusion criteria at the beginning of the Oral Health Component
OHE_N72
Instruction: Record the serious medical condition discovered during the dental examination.
- Oral lesion
- Severe acute infection
- Other – Specify (insert condition to a maximum of 80 characters)
Note: Don’t Know and Refused are not allowed. All respondents found to have a serious medical condition that requires immediate attention discovered during the dental examination [OHE_N71 = 1]
OHE_END
The Lab Component does not require the presence of the respondent but is completed at the mobile clinic during, or soon after, the respondent’s visit. (Mobile clinic/lab components include initial blood and urine splitting, and complete blood count (CBC) processing.) Respondent specimens are sent for further analysis to three reference laboratories specializing in nutritional analysis, environmental contaminant analysis and infectious disease analysis. The National Microbiology Laboratory in Winnipeg is the storage laboratory for the Canadian Health Measures Survey.
Respondents who so request receive a report of their blood and urine tests 8-12 weeks after the clinic visit.
Once all of the clinic components have been completed, the following additional variables are calculated:
- musculoskeletal fitness norms for respondents aged 15 to 69
- back fitness norms for respondents aged 15 to 69.
Then a “Report of Measurements” is produced for the respondent.
Instruction: Print the Report of Measurements (sample attached in Appendix V) and associated letters to physicians for urgent conditions (samples attached in Appendix VI) for blood pressure, and oral health.
RM_END
To be completed by all respondents.
ECI_R01
Before you leave, we have a few administrative questions.
[WARNING: “The respondent should speak with the dentist before leaving the clinic.”]
Note: The above text will only appear if an oral health results report has been generated in the report of measurements component, inform the respondent that he must speak to the dentist before leaving the clinic.
ECI_END
Data linking
ECQ_R11
We are seeking your permission to combine information collected during the Canadian Health Measures Survey with health information from your [provincial/territorial] ministry of health or cancer/vital statistics registrars. This would include information on past and continuing use of services provided at hospitals, clinics, and doctor’s offices, or other health services provided by the [province/territory], but it does not include personal medical information held by your doctor.
ECQ_R12
Information collected during the Canadian Health Measures Survey will include:
- the responses you provided to the interviewer at your home
- the results of the physical measures tests that you did today
- [the information that will result from your activity monitor, which you will return to Statistics Canada]
- [the results of tests to be done in the future on your blood and urine samples, collected today]
- [the results of tests to be done in the future on your DNA sample]
ECQ_Q13
The linkage will be done by Statistics Canada, and the results will be used for statistical purposes only.
Do we have your permission?
- Yes
- No (Go to ECQ_R21)
Don’t Know, Refused (Go to ECQ_R21)
All respondents
ECQ_Q14
Having a [provincial/territorial] health number will assist Statistics Canada in linking the survey data to the [provincial/territorial] health information.
Do you have a(n) [province/territory name] health number?
- Yes (Go to ECQ_Q16)
- No
Don’t Know, Refused (Go to ECQ_R21)
All respondents
ECQ_Q15
For which [province/territory] is your health number?
10. Newfoundland and Labrador
11. Prince Edward Island
12. Nova Scotia
13. New Brunswick
24. Quebec
35. Ontario
46. Manitoba
47. Saskatchewan
48. Alberta
59. British Columbia
60. Yukon
61. Northwest Territories
62. Nunavut
88. Does not have a [provincial/territorial] health number
Don’t Know, Refused (Go to ECQ_R21)
All respondents
ECQ_Q16
What is your health number?
Instruction: Enter a health number. Do not insert blanks, hyphens or commas between the numbers. (insert respondent Health Number)
All respondents who have a health number
Data sharing
ECQ_R21
Statistics Canada would like to share the information collected during the Canadian Health Measures Survey with Health Canada and the Public Health Agency of Canada. [Your name, address, telephone number and health number / Your name, address and telephone number] will not be shared.
ECQ_Q22
Health Canada and the Public Health Agency of Canada will keep the information confidential, and use it for statistical purposes only.
Do you agree to share the information?
- Yes
- No
All respondents
ECQ_END
Appendices
Welcome
CONFIDENTIAL WHEN COMPLETED
Your participation is important to us. Please check all of the information shown below to ensure it is accurate. If you find mistakes, please tell the Coordinator or write the correct information on this form and return it to the Coordinator
- Date (yyyy/mm/dd): 2007/03/14
- Identification Number: 23456789
- Name: Jane Doe
- Date of birth (yyyy/mm/dd): 1958/02/10
- Sex: Female
- Preferred official language: English
- Corrections:
- Name:
- Date of birth:
- Sex:
- Preferred official language:
- For office use only:
Assent Form
Confidential when completed
- Date (yyyy/mm/dd): 2007/03/14
- Identification Number: 23456789
- Name: Jane Doe
- Age at clinic exam: 4
- Gender: Female
The clinic portion of this survey has some tests for you to do. We also want to keep some of your blood and urine for tests that will happen later.
You do not have to do any part of the survey that you do not want to do.
If you want to take part in this survey, write or print your name below.
Name of respondent
- Signature of participant
- Name of witness (please print)
- Signature of witness
- For office use only:
Consent Form
Confidential when completed
- Date (yyyy/mm/dd): 2007/03/14
- Identification Number: 23456789
- Name of participant: John Doe
- Age at clinic exam: 10
- Gender: Male
I have read and understood the information provided to me in the Information and Consent Booklet for the Canadian Health Measures Survey. By marking the boxes below and signing this form, I am choosing to consent (“Yes”) or not consent (“No”) to the following for [respondent’s first name]:
- participating in the physical measure tests, including providing samples of his blood and urine
- receiving a copy of his Report of Laboratory Tests
- storage of his blood and urine for use in future health studies
I have had time to decide on allowing [respondent’s first name] to participate in the clinic portion of the survey. I understand that even though I have consented to some or all of the items on this form, I can still withdraw [respondent’s first name] from any part of this survey or subsequent studies at any time until he reaches 14 years of age. From that point onwards, [respondent’s first name] can decide to withdraw.
- Name of parent/guardian (please print)
- Signature of parent/guardian
- Name of witness (please print)
- Signature of witness
- For office use only:
Consent Form
Confidential when completed
- Date (yyyy/mm/dd): 2007/03/14
- Identification Number: 23456789
- Name: Peter Doe
- Age at clinic exam: 15
- Gender: Male
I have read and understood the information provided to me in the Information and Consent Booklet for the Canadian Health Measures Survey. By marking the boxes below and signing this form, I am choosing to consent (“Yes”) or not consent (“No”) to the following:
- participating in the physical measure tests, including providing samples of my blood and urine
- receiving a copy of my Report of Laboratory Tests
- allowing Statistics Canada to test my blood for the Hepatitis B and C viruses and to contact me, as well as the appropriate provincial authorities, if the results are positive
- storage of my blood and urine for use in future health studies
I have had time to decide on participating in the clinic portion of the survey. I understand that even though I have consented to some or all of the items on this form, I can still withdraw from any part of this survey or subsequent studies at any time.
- Name of respondent
- Signature of participant
- Name of witness (please print)
- Signature of witness
- For office use only:
Consent Form
Confidential when completed
- Date (yyyy/mm/dd): 2007/03/14
- Identification Number: 23456789
- Name: Susan Doe
- Age at clinic exam: 22
- Gender: Female
I have read and understood the information provided to me in the Information and Consent Booklet for the Canadian Health Measures Survey. By marking the boxes below and signing this form, I am choosing to consent (“Yes”) or not consent (“No”) to the following:
- participating in the physical measure tests, including providing samples of my blood and urine
- receiving a copy of my Report of Laboratory Tests
- allowing Statistics Canada to test my blood for the Hepatitis B and C viruses and to contact me, as well as the appropriate provincial authorities, if the results are positive
- storage of my blood and urine for use in future health studies
- storage of my DNA for use in future health studies
I have had time to decide on participating in the clinic portion of the survey. I understand that even though I have consented to some or all of the items on this form, I can still withdraw from any part of this survey or subsequent studies at any time.
- Name of respondent
- Signature of participant
- Name of witness (please print)
- Signature of witness
- For office use only:
PAR-Q & YOU (A questionnaire for people aged 15 to 69)
Regular physical activity is fun and healthy, and increasingly more people are starting to become more active very day. Being more active is very safe for most people. However, some people should check with their doctor before they start becoming much more physically active.
If you are planning to become much more physically active than you are now, start by answering the seven questions in the box below. If you are between the ages of 15 and 69, the PAR-Q will tell you if you should check with your doctor before you start. If you are over 69 years of age, and you are not used to being very active, check with your doctor.
Common sense is your best guide when you answer these questions. Please read the questions carefully and answer each one honestly: check Yes or No.
- Has your doctor ever said that you have a heart condition and that you should only do physical activity recommended by a doctor?
- Do you feel pain in your chest when you do physical activity?
- In the past month, have you had chest pain when you were not doing physical activity?
- Do you lose your balance because of dizziness or do you ever lose consciousness?
- Do you have a bone or joint problem (for example, back, knee or hip) that could be made worse by a change in your physical activity?
- Is your doctor currently prescribing drugs (for example, water pills) for your blood pressure or heart condition?
- Do you know any other reason you should not do physical activity?
If you answered Yes to one or more questions
Talk with your doctor by phone or in person Before you start becoming physically active or Before you have a fitness appraisal. Tell your doctor about the PAR-Q and which questions you answered Yes.
- You may be able to do any activity you want – as long as you start slowly and build up gradually. Or, you may need to restrict your activities to those which are safe for you. Talk with your doctor about the kinds of activities you wish to participate in and follow his/her advice.
- Find out which community programs are safe and helpful for you.
No to all questions
If you answered No honestly to all PAR-Q questions, you can be reasonably sure that you can:
- start becoming much more physically active – begin slowly and build up gradually. This is the safest and easiest way to go.
- take part in a fitness appraisal – this is an excellent way to determine your basic fitness so that you can plan the best way for you to live actively. It is also highly recommended that you have your blood pressure evaluated. If your reading is over 144/94, talk with your doctor before you can start becoming much more physically active.
Delay becoming more active:
- if you are not feeling well because of a temporary illness such as a cold or a fever – wait until you feel better; or
- if you are or may be pregnant – talk to your doctor before you start becoming more active.
Please note:
If your health changes so that you answer Yes to any of the above questions, tell your fitness or health professional. Ask whether you should change your physical activity plan.
Informed use of the PAR-Q:
The Canadian Society for Exercise Physiology, Health Canada, and their agents assume no liability for persons who undertake physical activity, and if in doubt after completing this questionnaire, consult your doctor prior to physical activity.
No changes permitted. You are encourages to photocopy the PAR-Q but only if you use the entire form.
Note:
If the PAR-Q is being given to a person before he or she participates in a physical activity program or a fitness appraisal, this section may be used for legal or administrative purposes. “I have read, understood and completed this questionnaire. Any questions I had were answered to my full satisfaction.”
- Name
- Signature
- Signature of parent
- Witness
- or Guardian (for participants under the age of majority)
Note:
This physical activity clearance is valid for a maximum of 12 months from the date it is completed and becomes invalid if your condition changes so that you would answer Yes to any of the seven questions.
- The appropriate predicted equation
- 6-7 years = Corey 1976
- Predicteds
- For respondents aged 6-7 the ‘Predicteds’ used are from ‘Corey 1976’
- Ethnic Group for COREY 1976
- Race adjustment Group
- White
- Black
- Hispanic
- Asian
- Other
- The appropriate predicted equation
- 8+ years = Hankinson (NHANES III)
- Predicteds
- For respondents 8 years and older, the ‘Predicteds’ used are from ‘Hankinson (NHANES III)
- Ethnic Group for HANKINSON (NHANES III):
- Race adjustment Group
- White
- Black
- Hispanic
- Asian
- Other
Report of measurements
Section A: Demographic information
- Date of appointment: 2007/03/14
- Name of respondent: John Doe
- Age of respondent at clinic exam: 50
- Gender of respondent: Male
Section B: Blood pressure and heart rate
- Resting Heart Rate: 85 bpm
- Average Systolic Blood Pressure: 145 mmHg
- Average Diastolic Blood Pressure: 112 mmHg
Your blood pressure today is high.YOU SHOULD SEE A DOCTOR WITHIN THE NEXT WEEK TO HAVE YOUR BLOOD PRESSURE RECHECKED.
Section C: Anthropometric measures
Body measurements
- Standing Height:
- Sitting Height:
- Weight:
- Waist Circumference:
- Hip Circumference:
- Waist-to-Hip Ratio: 1.04
- Sum of five skinfold measurements: Not measured
Composite measures
- Body Mass Index (BMI): 35.32 kg/m2
Your body mass index score classifies you as obese. If you are very obese, you may have a very high risk of developing health problems. For an accurate classification, BMI should be interpreted along with other body composition scores.
Body Composition:
(calculated based on waist circumference, sum of five skinfold measurements, and BMI)
Your body composition falls within a range that is generally associated with considerable health risk. We suggest that you see a doctor or regulated health professional to follow-up on your results.
Section D: Lung function (Spirometry)
- Forced Vital Capacity (FVC):
- Measured: 3.90 L
- Predicted: 5.09 L
- % Predicted: 76.6 %
- Forced Expiratory Volume (FEV1):
- Measured: 2.25L
- Predicted: 3.74L
- % Predicted: 60.2 %
- FEV1/FVC:
Your lung function score today is outside the normal range for your age and sex. We suggest that you see a doctor or regulated health professional to follow-up on your results.
Section E: Fitness and strength tests
Modified Canadian aerobic fitness test (mCAFT)
- Aerobic Fitness Score: Not calculated
Grip strength
- Total hand grip strength: 77 kg
- Your score for your age and sex is fair.
Sit and reach
- Distance reached: 7.5 cm
- Your score for your age and sex is poor.
Partial curl-ups
- Number of partial curl-ups completed: 0
- Your score for your age and sex is poor.
Composite measures
Musculoskeletal Fitness:
(calculated based on grip strength, sit and reach, and partial curl-ups)
Your musculoskeletal fitness falls within a range that is generally associated with considerable health risk. We suggest that you see a doctor or regulated health professional to follow-up on your results.
Back Fitness:
(calculated based on waist circumference, sit and reach, and partial curl-ups)
Your back fitness falls within a range that is generally associated with considerable back health risk. We suggest that you see a doctor or regulated health professional to follow-up on your results.
Section F: Oral health
During the oral health examination today, the dentist had some concerns about the health of your teeth and/or mouth. You are encouraged to visit a dental professional within a week.
Blood pressure test results report
- Date of appointment: 2007/03/14
- Name: John Doe
- Result of blood pressure test: 145 / 112 mmHg
Your blood pressure today is high. You should see a doctor within the next week to have your blood pressure rechecked.
Note: Based on a report by the Canadian Coalition for High Blood Pressure Prevention and Control, 1994
To whom it may concern:
John Doe was recently a participant in the Canadian Health Measures Survey (CHMS) conducted by Statistics Canada. The CHMS is a national survey that collects information about the general health and health behaviours of Canadians. The information gathered through direct measures of health is essential to evaluate the extent of such major health concerns as diabetes, obesity, hypertension and cardiovascular disease. The results from this survey will also provide researchers with important and precise information about health issues that affect all Canadians.
The survey was conducted in two phases: an interview at the household and a visit to a CHMS clinic. At the clinic, fully trained health professionals took direct measures of health such as blood pressure, height, weight, spirometry, blood and urine samples, physical fitness tests and an oral health examination.
At the clinic a CHMS health measures specialist performed blood pressure testing using an automated blood pressure device (BPTru). After five minutes of quiet rest in a screening room, six blood pressure measurements were taken at one minute intervals, and the average of the last five measurements was calculated.
The tests performed as part of the CHMS are not intended to be used for diagnostic purposes. We have recommended that John Doe follow-up on any abnormal test results with a doctor or other regulated health professional.
If you have any questions about the CHMS please contact us, toll-free, at 1-888-253-1087, or visit our website at http://www.statcan.ca.
Sincerely,
CHMS Health Measures Specialist
Oral examination results report
- Date of appointment: 2007/03/14
- Name: John Doe
- Result of oral examination: a severe acute infection
To whom it may concern:
John Doe was recently a participant in the Canadian Health Measures Survey (CHMS) conducted by Statistics Canada. The CHMS is a national survey that collects information about the general health and health behaviours of Canadians. The information gathered through direct measures of health is essential to evaluate the extent of such major health concerns as diabetes, obesity, hypertension and cardiovascular disease. The results from this survey will also provide researchers with important and precise information about health issues that affect all Canadians.
The survey was conducted in two phases: an interview at the household and a visit to a CHMS clinic. At the clinic, fully trained health professionals took direct measures of health such as blood pressure, height, weight, spirometry, blood and urine samples, physical fitness tests and an oral health examination.
At the clinic a CHMS dentist performed an oral examination and noticed a severe acute infection in John Doe ’s mouth. This is a serious medical condition requiring immediate attention from either a dental or a medical professional.
The tests performed as part of the CHMS are not intended to be used for diagnostic purposes. We have recommended that John Doe follow-up on any abnormal test results with a doctor or other regulated health professional.
If you have any questions about the CHMS please contact us, toll-free, at 1-888-253-1087, or visit our website at http://www.statcan.ca.
Sincerely,
CHMS Dentist
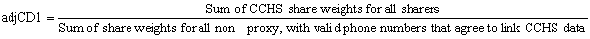
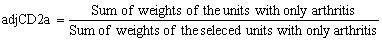
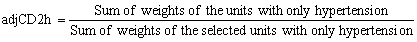
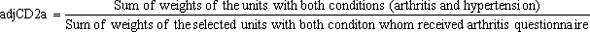
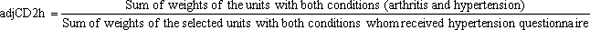
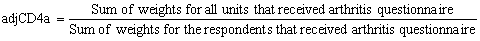
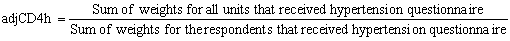
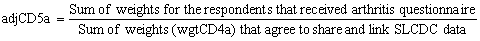
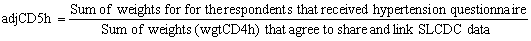
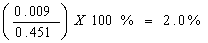
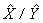 are obtained by:
are obtained by: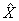 );
);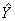 ); then
); then